Characterization of nHAP, nHAP/PLGA, and MINO@PLGA
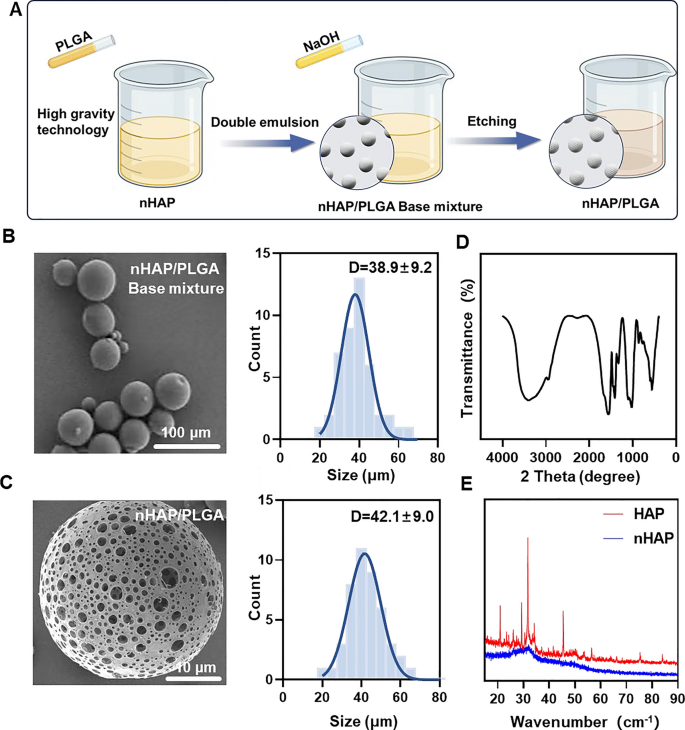
MINO was loaded onto porous nHAP/PLGA microspheres in a easy, environment friendly, cost-effective, and environmentally-friendly method to synthesize MINO@PLGA. It features by enhancing TGF-β expression, lowering ocular irritation, accelerating corneal epithelial therapeutic, and inhibiting neovascularization (Graphical Summary). Initially, a nano-hydroxyapatite (nHAP) with ultra-small measurement and excessive dispersion stability is ready utilizing ultracentrifugation know-how adopted by combining it with PLGA to kind a base combination. This combination is then etched with NaOH to provide the ultimate nHAP/PLGA microspheres (Fig. 1A). SEM reveals that nHAP/PLGA base combination has a great dispersibility and spherical form with particle measurement of 38.9 ± 9.2 μm (Fig. 1B). In distinction, the etched nHAP/PLGA microspheres exhibit a porous construction appropriate for loading MINO with particle measurement of 42.1 ± 9.0 μm (Fig. 1C). The FT-IR spectrum of nHAP (Fig. 1D), shows peaks for O–H at 3300 cm−1 to 3400 cm−1 and 1500 cm−1 to 1600 cm−1. Peaks for P-O are evident at 1000 cm−1 to 1100 cm−1 and 500 cm−1 to 600 cm−1. This means that the product is hydroxyapatite. X-ray diffraction (XRD) signifies that nHAP has decrease diffraction peaks in comparison with HAP, suggesting enhanced crystalline stability within the synthesized nHAP (Fig. 1E).
Synthesis and characterizations of nHAP/PLGA. A Schematic illustration on the fabrication of nHAP/PLGA. B, C SEM of nHAP/PLGA base combination and nHAP/PLGA with the corresponding measurement of nHAP/PLGA base combination and nHAP/PLGA. D FT-IR characterization of nHAP. E XRD characterization of nHAP and HAP
Incorporating nHAP retains the scaffold’s biocompatibility and improves the biodegradability of the microspheres. Furthermore, the XRD of nHAP shows an amorphous construction, indicating enhanced drug loading and sustained-release capabilities. The drug-loading capability of nHAP/PLGA microspheres, measured at an absorbance of 360 nm, is roughly 50%. Biodegradable polymers have garnered rising curiosity on account of their biocompatibility, non-toxicity, and numerous properties. PLGA stands out as some of the well known biodegradable polymers. Notably, it has obtained approval from the FDA for functions in drug supply, diagnostics, and varied fields of scientific and fundamental science analysis, encompassing heart problems, most cancers, vaccines, and tissue engineering [17]. An in vivo ocular anti-inflammatory research performed in rabbit eyes confirmed the superior efficacy of drug-loaded nanoparticles compared to drug options [18]. Two analysis groups have additionally designed PLGA nanoparticles loaded with tacrolimus for topical ocular instillations [19]. Alshamsan et al. developed PLGA-tacrolimus nanoparticles utilizing the emulsification-diffusion technique, demonstrating a big enhancement in ocular bioavailability by means of the entrapment of tacrolimus by nanoparticles [20]. Equally, Benita et al. developed PLGA-tacrolimus nanoparticles utilizing a well-established solvent displacement technique. It is value noting that repeated ocular instillations of those nanoparticles in rat eyes resulted in elevated tacrolimus ranges inside the eye, whereas plasma concentrations remained low [19].
Moreover, the scale of micro-particles performs a vital function within the drug launch price. A discount in particle measurement results in a rise in floor space and mass switch for a hard and fast mass of drug and polymer [21]. Lyu et al. [9] encapsulated bevacizumab (BEV) inside the pores of mesoporous silica nanoparticles (MSNs), forming BEV@MSN nanoparticles with a median diameter of 39.3 ± 5.3 nm. In the meantime, Zhang et al. [22] ready PLGA-bevacizumab nanoparticles with a hydrodynamic diameter of roughly 133 nm, reaching an entrapment effectivity and loading effectivity of round 80.0% and 6.8%, respectively. On this research, the loading of MINO into porous nHAP/PLGA microspheres, with a drug loading capability of roughly 50%, may improve the bioavailability and increase the anti-angiogenic effectivity of MINO. Furthermore, MINO@PLGA nanoparticles exhibit a spherical form with a clean floor, which may reduce susceptibility to shear forces and facilitate efficient interactions with cell surfaces, thereby rising mobile uptake. Consequently, PLGA-mediated MINO supply guarantees to increase the residence time of MINO in vivo, resulting in sustained concentrations and improved drug bioavailability.
MINO@PLGA microspheres launch, degradation, permeation and inhibit cell migration
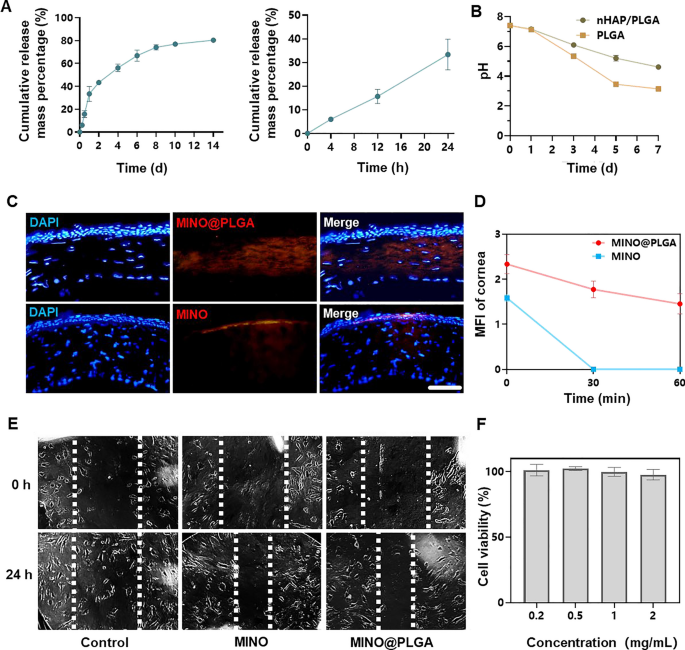
The cumulative launch of MINO from PLGA microspheres elevated over time, reaching roughly 80% by day 14 (Fig. 2A). This sustained launch sample means that the microspheres present a protracted therapeutic impact, doubtlessly countering the exercise of corneal neovascularization over an prolonged interval. The pH of PLGA microspheres declined inside 7 days, indicating the degradation of PLGA microspheres would possibly end in an acidic setting (Fig. 2B). Nevertheless, because of the irritant nature of low pH on eyes, there are limitations to the usage of PLGA microspheres. In distinction, nHAP/PLGA demonstrated a extra secure pH post-degradation in comparison with PLGA, suggesting higher tissue compatibility. Mixed photographs revealed that MINO@PLGA successfully remained inside the corneal epithelium, whereas standalone MINO did not penetrate the corneal epithelium (Fig. 2C). This means that MINO@PLGA enhances drug contact time with ocular tissues, offering a managed and guarded supply of medicine, doubtlessly extending therapeutic results. Pharmacokinetic knowledge derived from fluorescence depth measurements of the cornea (Fig. 2D) present the sustained launch profile of our formulation. On the 60-min mark, a big distinction in fluorescence depth was noticed between the MINO and MINO@PLGA teams. This distinction signifies that the MINO@PLGA microspheres not solely facilitate enhanced corneal penetration but in addition contribute to a protracted drug contact time with ocular tissues. Particularly, whereas the typical retention time of ordinary ocular medicines on the attention floor is 2–3 min [23], our MINO@PLGA formulation exhibited extended residence time. Such knowledge underscore the efficacy of the MINO@PLGA system in sustaining sustained drug concentrations on the goal website, thus doubtlessly extending therapeutic results.
Minocycline microspheres launch, degradation, permeation and inhibit cell migration. A Minocycline microspheres launch profile. B Degradation of nHAP/PLGA over time with pH. C Fluorescent retention statement of e minocycline or MINO@PLGA in cornea (scale bar: 100 μm). D Adjustments within the cornea common fluorescence depth of drug after eye drop at 0, 30, and 60 min (n = 3). E Scratch therapeutic assay of HUVECs handled with minocycline (1 mg/mL) or MINO@PLGA (1 mg/mL). Consultant photographs of the scratch hole have been captured at 0 h and 24 h. F Impact of MINO@PLGA on viability of HUVECs
This means that MINO@PLGA microspheres, with their sustained launch and enhanced corneal penetration, contribute to extended drug contact with ocular tissues. Each MINO and MINO@PLGA exhibited inhibitory results on HUVEC migration after 24 h, with MINO@PLGA demonstrating a barely stronger impact (Fig. 2E, F). This means that encapsulated MINO retains its bioactivity, and the sustained launch from PLGA microspheres might improve its inhibitory results, making it a possible superior agent for wound therapeutic or anti-angiogenic functions.
The severity of corneal alkali burns and the extent of tissue harm can considerably impression the timing and depth of the inflammatory response. In sure cases, irritation and CoNV might persist for an prolonged length, notably when the harm is extreme or sophisticated by points reminiscent of an infection. The vast majority of present therapies exhibit optimum efficacy inside a comparatively restricted temporal window following the initiation of CoNV, with diminishing effectiveness as neovascularization matures and stabilizes [2]. The BEV@MSN nanoparticles, as ready by Lyu et al. [9], exhibited a launch length of as much as 4 weeks, with solely about 27% and 33% launched on the primary and second days, respectively, adopted by a virtually linear launch over the following three weeks. In distinction, the PLGA-bevacizumab nanoparticles ready by Zhang et al. [22] demonstrated a special launch profile, with greater than 40% of bevacizumab being launched inside the first 2 h, and a further approximate 40% launched within the subsequent 7 days, adopted by a slower launch extending as much as 21 days. MINO@PLGA extends the half-life of MINO and achieves sustained launch from PLGA nanoparticles in a secure, managed method. This sustained launch has the potential to constantly counteract the exercise of corneal neovascularization. MINO@PLGA retains extra successfully within the cornea, prolonging drug contact time with ocular tissues, delivering medication to a selected tissue website in a managed method, defending medication from degradation. This can be attributed to the nice water solubility of the MINO@PLGA microspheres and the superior liposolubility of MINO inside the tetracycline class, enabling it to penetrate the corneal epithelium and attain the corneal stroma [24].
Irregular endothelial cell proliferation, migration, and tube formation is crucial for angiogenic results [25]. MINO@PLGA would possibly exhibit a stronger inhibitory impact on HUVECs migration, doubtlessly making it a superior agent for wound therapeutic or anti-angiogenic functions [26]. As well as, insufficient corneal penetration and the speedy clearance of medicine from the ocular floor considerably undermine the therapeutic efficacy of topical eye drops for the remedy of ocular ailments. Consequently, frequent administration of topical medication or high-dose systemic remedy is commonly required (30–60 mg/kg MINO every day) [15], which can end in diminished affected person compliance and compromised therapeutic effectiveness. In our research, the dose and frequency of drug administration could possibly be decreased.
Analysis of anti-CoNV impact
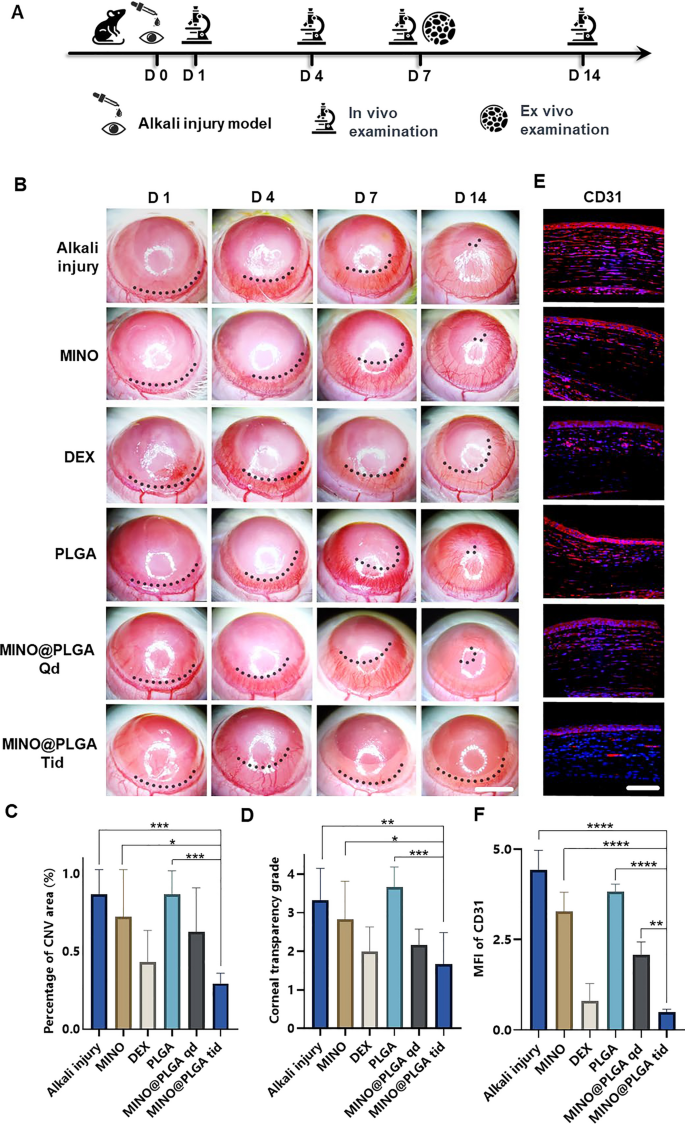
The corneal alkali burn mannequin was established on day 0, adopted by steady statement for 14 days post-operation. Slit-lamp in vivo examinations have been performed on days 1, 4, 7, and 14, with a subset of rats sacrificed for ex vivo evaluation on day 7 (Fig. 3A). The inhibitory impact of MINO@PLGA on CoNV was evaluated by constantly observing and evaluating the CoNV space share and corneal transparency on the similar corneal location between the MINO@PLGA (Qd and Tid) teams and the management group. From day 1 and 4 post-alkali burn, the neovascular buds within the MINO@PLGA (Qd and Tid) teams confirmed similarity to these within the management group (p > 0.05). From day 4 to day 14, corneal neovascularization within the group handled with MINO@PLGA Tid exhibited a lower. In distinction, CoNV development within the management group was notably strong, and it’s time-dependent (Fig. 3B). The neovascular space ratio was additional quantified. As proven in Fig. 3C, on day 14, the CoNV space for the MINO@PLGA Tid group was the smallest at 29.40% ± 6.55%. These values have been decrease than these within the management group (86.81% ± 15.71%), MINO group (72.42% ± 30.15%), PLGA group (86.87% ± 14.94%) (p < 0.05), and the DEX group (43.23% ± 20.35%), MINO@PLGA Qd group (62.78% ± 28.12%) (p > 0.05). Quantification confirmed the visible observations; the discount of CoNV in corneas handled with MINO@PLGA suggests its potential in successfully managing post-injury corneal neovascularization. Corneal transparency, reflecting the edematous state, harm, and inflammatory situation, assorted amongst therapies. MINO@PLGA (Qd and Tid) therapies led to decreased opacity (p < 0.05), suggesting improved wound therapeutic and attenuation of irritation essential for sustaining corneal readability (Fig. 3D). Samples handled with MINO@PLGA (Qd and Tid) exhibited decreased CD31 fluorescence, indicating diminished vascular formation (Fig. 3E). In comparison with different samples (apart from the DEX group), these handled with MINO@PLGA, particularly Tid, demonstrated a notably decrease MFI of CD31 (Fig. 3F) (p < 0.05).
MINO@PLGA inhibits corneal neovascularization and reduces haze. A Examination time circulate after corneal alkali burn. B The corneal neovascularization was examined at 1, 4, 7, 14 days after the corneal alkali burn (scale bar: 3 mm), and the quantified corneal neovascularization of 14 days is proven in (C) (n = 6). D Corneal opacity at 14 days between teams (n = 6). E CD31 immunofluorescence at 7 days. F MFI of CD31 (scale bar: 100 μm, n = 3). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
Minocycline, a tetracycline by-product, possesses the power to penetrate the blood–mind barrier and serves as a broad-spectrum antibiotic with demonstrated anti-inflammatory, anti-apoptotic, and antioxidant properties [27]. Its in depth utilization consists of the remedy of respiratory infections, reproductive tract infections, pores and skin infections, and suppurative infections [28]. Our earlier research demonstrated minocycline has neuroprotective impact through its anti-inflammation mechanism [7]. Furthering the understanding of minocycline’s therapeutic results, analysis by Yuan et al. [29] revealed its important anti-inflammatory exercise in rat fashions of endotoxin-induced uveitis and retinal irritation. This was achieved primarily by means of the attenuation of LPS-induced expression of IL-1β and CCL-2. Earlier analysis [15] involving systemic administration of minocycline, notably by means of intraperitoneal injection in a mouse mannequin of alkali burn-induced corneal neovascularization, demonstrated a lower in CoNV to 73.03% ± 17.81%, in stark distinction to 97.43% ± 3.91% noticed within the PBS group on day 14. In our research, we noticed a markedly extra important discount within the CoNV space with the MINO@PLGA remedy—particularly, 29.40% ± 6.55% in comparison with 86.81% ± 15.71% within the management group on day 14. This discovering not solely underscores the effectiveness of our MINO@PLGA remedy in lowering CoNV but in addition extends earlier observations by exhibiting that minocycline, when delivered successfully, can considerably lower the extent of CoNV. These outcomes solidify minocycline’s potential as a precious therapeutic agent within the remedy of CoNV.
The diminished opacity with MINO@PLGA remedy suggests improved wound therapeutic and attenuation of irritation, which is essential for sustaining corneal readability [30]. The decrease MFI of CD31 in MINO@PLGA-treated samples additional quantifies its anti-angiogenic properties [31], suggesting a possible much like the anti-inflammatory drug dexamethasone in managing corneal neovascular ailments, which was superior to MINO alone [32]. And In comparison with the MINO@PLGA Qd group, the MINO@PLGA Tid group demonstrates the power to keep up a enough drug focus, thus bettering efficacy within the remedy of CoNV and lowering opacity.
Harm therapeutic
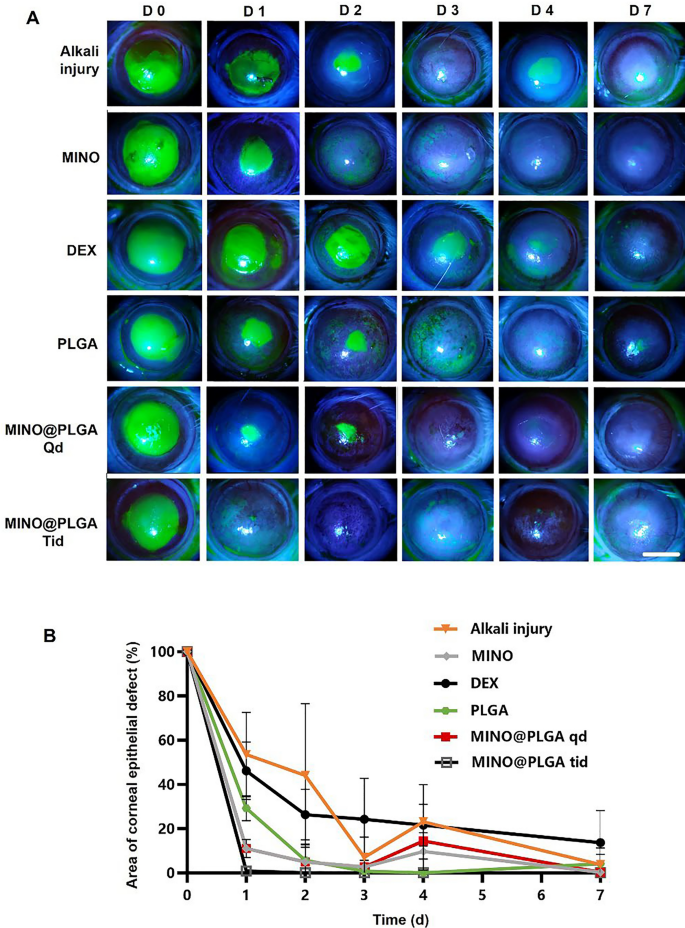
Fluorescein sodium staining was employed to visualise the development of corneal epithelial accidents throughout all teams (Fig. 4A). Notably, each the MINO group and the MINO@PLGA group exhibited no indicators of nonhealing in corneal epithelial harm restore, highlighting the efficacy of MINO and MINO@PLGA in selling harm restore and controlling irritation. Determine 4B illustrates the comparative evaluation of epithelial therapeutic charges amongst completely different remedy teams. MINO@PLGA (Qd and Tid) demonstrated a notably sooner wound therapeutic price and higher epithelial operate restoration in comparison with different therapies, together with the DEX group. The MINO@PLGA Tid group, specifically, exhibited environment friendly promotion of epithelial therapeutic post-alkali burn. Corneas handled with MINO@PLGA Tid demonstrated speedy reductions in epithelial defect areas, with virtually full therapeutic noticed by day 7. In distinction, the Alkali harm group and the DEX group confirmed slower corneal epithelial therapeutic and longer therapeutic instances (p < 0.05).
Adjustments within the corneal epithelial harm space over time in numerous teams after alkali burn. A Typical fluorescein sodium staining of the corneal epithelium was examined at 0, 1, 2,3, 4, 7 days after the corneal alkali burn (scale bar: 3 mm), the share of corneal epithelial defect space over time is proven in (B) (n = 6)
Corneal keratolysis, typically related to heightened irritation and impaired re-epithelialization, necessitates methods that reduce irritation and expedite re-epithelization to forestall problems [33, 34]. It’s recognized that glucocorticoids, reminiscent of dexamethasone (DEX), can impede wound therapeutic by hindering cell migration, as demonstrated in earlier research [35, 36]. Our findings align with this notion, exhibiting that the DEX group exhibited delayed corneal epithelial therapeutic in comparison with different therapies.
In distinction, each the MINO group and the MINO@PLGA group displayed environment friendly corneal epithelial harm restore, underscoring some great benefits of MINO and MINO@PLGA in selling harm restore whereas successfully controlling irritation. Fluorescein sodium staining confirmed the progress of corneal epithelial accidents, offering a visible illustration of the optimistic outcomes related to MINO and MINO@PLGA therapies.
The outcomes from Fig. 4B additional emphasize the prevalence of MINO@PLGA (Qd and Tid) in selling epithelial therapeutic in comparison with different therapies, together with the DEX group. The sooner restoration noticed within the MINO@PLGA Tid group means that frequent utility of this formulation might supply superior remedy results in selling wound therapeutic after alkali harm. This aligns with the essential function of accelerated re-epithelialization in advancing total wound therapeutic [37]. These findings collectively spotlight the potential scientific significance of MINO@PLGA in managing acute ocular alkali burns by facilitating immediate corneal epithelial therapeutic whereas minimizing inflammation-related problems.
Immunofluorescent staining
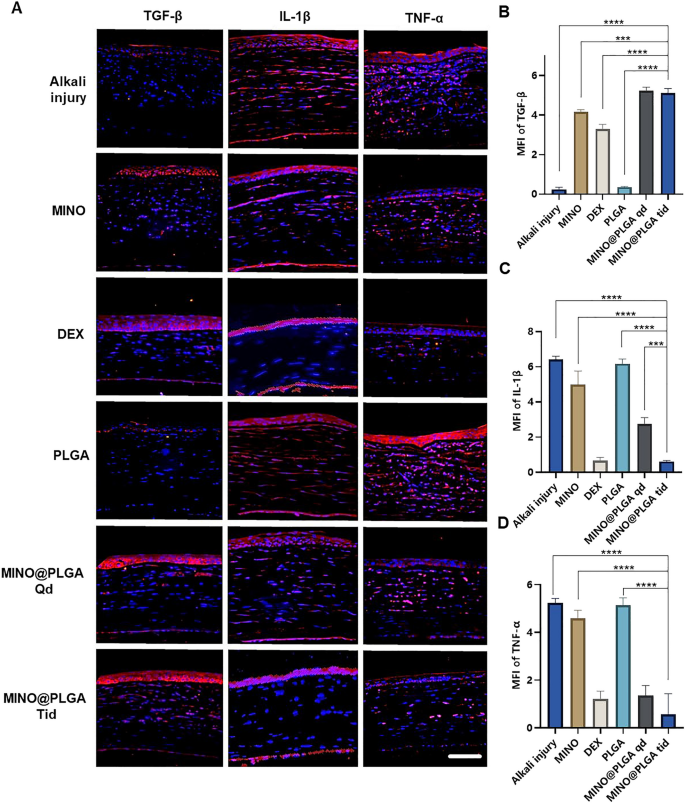
Photographs reveal completely different ranges of corneal TGF-β and inflammatory components IL-1β and TNF-α in varied remedy teams on day 7 post-alkali burn. Within the management group, the expression degree of TGF-β within the corneal epithelium is decreased, whereas ranges of IL-1β and TNF-α within the corneal stroma are elevated. With dexamethasone, MINO, and MINO@PLGA therapies, there’s an elevated expression of TGF-β, whereas inflammatory components IL-1β and TNF-α present various levels of lower (Fig. 5A). Semi-quantitative evaluation outcomes (Fig. 5B) point out that the expression of TGF-β was highest within the MINO@PLGA Qd and MINO@PLGA Tid teams (5.23 ± 0.18, 5.12 ± 0.23 respectively), adopted by the MIMO group (4.16 ± 0.12). The Dexamethasone group confirmed a slight lower (3.23 ± 0.25), whereas the Alkali harm and PLGA teams exhibited the bottom values (0.24 ± 0.10 and 0.35 ± 0.03 respectively). The expression of IL-1β and TNF-α was lowest within the MINO@PLGA Tid group (0.61 ± 0.04 and 0.57 ± 0.50, respectively) and confirmed statistically important variations when in comparison with the management group (p < 0.0001) (Fig. 5C, D). The discount of inflammatory within the cornea was additionally evidenced by H&E staining of corneal tissue (Extra file 1: Fig. S1). This means that MINO@PLGA would possibly suppress CoNV by means of the downregulation of inflammatory components IL-1β and TNF-α through the TGF-β pathway.
Throughout alkali burns, the corneal epithelium sustains harm, leading to a deficiency of pluripotent limbal stem cells and an imbalance between angiogenic stimulators and inhibitors. This imbalance can result in the over-proliferation and migration of capillary endothelial cells into the broken cornea [38]. The corneal epithelium performs a pivotal function within the mechanism of neovascularization. The expression of inflammatory components induced by TGF-β exerts anti-neovascular results [39]. TGF-β has additionally been reported to stimulate the transdifferentiation of corneal stromal cells into myofibroblasts, thereby rising corneal opacity [40]. Pruzanski et al. [41] demonstrated that minocycline inhibits phospholipase A2 (PLA2) and interacts with the substrate to attenuate the inflammatory response in sufferers with rheumatoid arthritis. Within the context of irritation, glial cells, each astrocytes and microglia, play pivotal roles [42]. Current research have proven that minocycline mitigates the event of ache hypersensitivity by inhibiting microglial activation, lowering proinflammatory cytokine expression in each inflammatory and neuropathic ache, and downregulating the manufacturing of proinflammatory cytokines, reminiscent of TNF-α, IL-1β, and IL-6 [43]. Moreover, minocycline was discovered to inhibit p38 MAPK in microglia, particularly focusing on this pathway. P38 MAPK is understood to mediate inflammatory responses in varied cell sorts, together with microglia.
With the progressive elucidation of minocycline’s anti-inflammatory results and underlying mechanisms, quite a few research have demonstrated its multifaceted properties in varied animal fashions of neuronal degenerative ailments. Minocycline reveals antimicrobial, anti-inflammatory, anti-apoptotic, and neuroprotective traits in situations reminiscent of Parkinson’s illness, a number of sclerosis, Alzheimer’s illness, Huntington’s illness, amyotrophic lateral sclerosis, and retinitis pigmentosa [44, 45]. Scholz et al. [46] demonstrated that minocycline supplies safety towards retinal harm in a mouse mannequin of acute retinal degeneration induced by white-light irradiation. Administration of minocycline led to the inhibition of inflammatory components, together with CCL2, IL-6, and iNOS, and resulted within the suppression of pro-inflammatory responses and neurotoxicity in microglia, whereas selling the survival of photoreceptors. On this research, we now have demonstrated, for the primary time, that minocycline, through the TGF-β pathway, downregulates the inflammatory components IL-1β and TNF-α to successfully suppress CoNV.
Biocompatibility
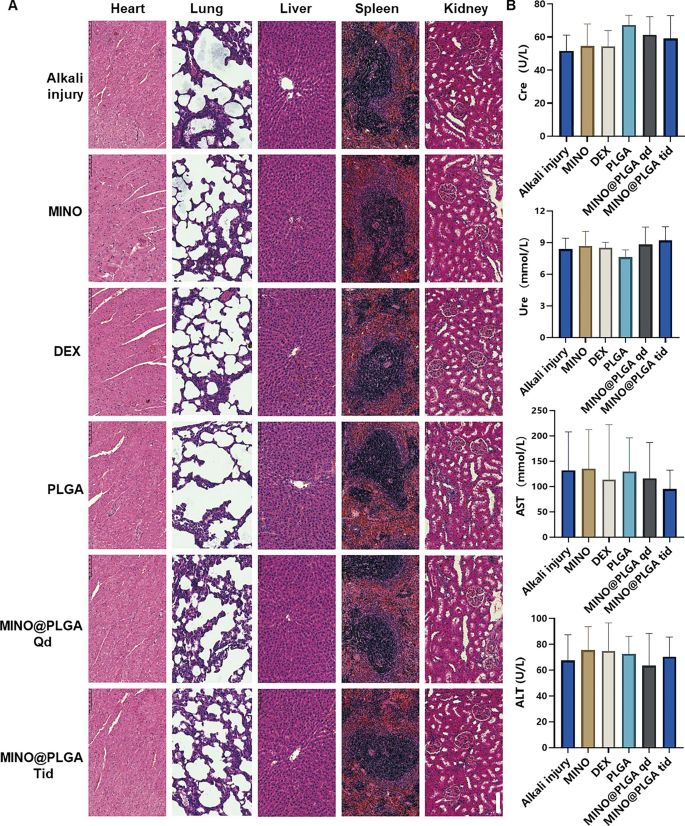
To evaluate the biosafety of MINO@PLGA, histological examination of main organ tissues (Coronary heart, lung, liver, spleen, kidney) was carried out utilizing H&E staining (Fig. 6A). The pictures throughout all remedy teams, together with MINO@PLGA therapies, revealed no overt indicators of organ harm. This means a good security profile for MINO@PLGA, with no discernible pathological modifications in main organs following its use post-alkali burn. Biochemical parameters from blood samples, together with Creatinine (Cre), Urea (Ure), Alanine aminotransferase (ALT), and Aspartate aminotransferase (AST), have been analyzed to additional consider the protection of MINO@PLGA (Fig. 6B). The information indicated that these parameters remained inside regular reference ranges throughout all remedy teams. The upkeep of regular ranges means that MINO@PLGA doesn’t adversely impression renal or liver operate, reinforcing its security as a remedy possibility following alkali burns.
Analysis of MINO@PLGA security on organ histopathology and systemic blood parameters. A H&E of main organs in rats from completely different teams at 7 days after corneal alkali burn (scale bar: 100 μm). B Biochemical indicators within the orbital venous blood of rats from completely different teams at 7 days after alkali burn. Creatinine (Cre) reference vary: 19.43–64.97 mmol/L. Urea (Ure) reference vary: 2.08–7.75 mmol/L. Alanine aminotransferase (ALT) reference vary: 33.7–98.7 U/L. Aspartate aminotransferase (AST) reference vary: 69.7–322.9 U/L (n = 6)
The systemic administration of minocycline is related to varied frequent opposed unintended effects [47]. Severe opposed results embody hypersensitivity syndrome response, drug-induced lupus, idiopathic intracranial hypertension, and different autoimmune syndromes, which can result in deadly outcomes [43]. To boost therapeutic efficacy and obtain extremely localized results whereas minimizing the chance of unintended effects in comparison with systemic administration, the usage of domestically delivered drug-loaded biodegradable nanoparticles presents an interesting various [6]. Topical administration, necessitating a considerably decrease dosage than systemic routes, is favored for ophthalmic drug supply [48]. Native injections, reminiscent of subconjunctival or intravitreal, are invasive and thus reserved for administration by healthcare professionals. Our research emphasised the topical administration of eye drops, a patient-friendly technique that may be self-administered [49].