Synthesis of GOA
GOA supplies have been synthesized in response to the beforehand reported strategies (Fig. S1) [6] and have been characterised by 1H NMR spectroscopy. The profitable synthesis of the intermediates was evidenced by the modifications within the chemical shift (δ) values of attribute protons within the 1H NMR spectra (Fig. S2). Particularly, the chemical shift values of the methylene teams change from 2 H (CH2OH) at δ = 3.72 to 2 H (-COO-CH2) at δ = 4.36 and three.99, respectively, as a result of conversion of the neighboring teams to an ester bond, supporting the profitable prevalence of an esterification response. In the meantime, the looks of the attribute peaks assigned to oleic acid proves the profitable coupling of gemcitabine and oleic acid for GOA manufacturing by way of an esterification condensation. Moreover, the profitable preparation of GOA is additional supported by mass spectrometry, which reveals a molecular ion peak of 528 (M + H) (Fig. S3), according to the theoretically calculated relative molecular mass of GOA.
Preparation and characterization of GOA/miR nanocomplex
GOA/miR-NC nanocomplexes have been initially ready by way of a beforehand reported sequential denaturation annealing and nanoprecipitation methodology [6]. As a result of the hydrogen bonding interactions between GOA and miR enhance with the temperature elevation, GOA/miR-NC was ready by a programmable annealing process at a set temperature of 37℃. A molar focus ratio of GOA to miR-NC at 250:1 or greater values led to full inhibition of miR-NC immigration in a gel retardation assay (Fig. 1A Fig. S4) as a result of formation of a stabilized GOA/miR-NC nanocomplex. Notice that this ratio is far better than the beforehand reported stabilized ratio of 120:1 for GOA/miR-NC [6] seemingly as a result of totally different base sequences of used miRNA, implying the potential of modulating the supramolecular interactions between GOA and miR-NC by way of various the sequence and construction of miR-NC. Though environment friendly miRNA condensation was noticed at a molar ratio of 250:1, a molar ratio was additional optimized to afford a stabilized nanocomplex with a imply diameter smaller than 200 nm appropriate for an enhanced permeation and retention (EPR) effect-mediated passive concentrating on to HCC. The imply diameter of all ready GOA/miR-NC nanocomplex was decided by dynamic mild scattering (DLS), which confirmed a mean dimension of roughly 147.1 nm at a molar ratio of 250:1 (Fig. 1B). TEM visualization demonstrates a spherical form with a mean dimension of roughly 150 nm for GOA/miR-NC nanocomplex (Fig. 1C).
The colloidal stability, together with storage stability, dilution, and media stability of GOA/miR-NC nanocomplex ready at a molar ratio of 250:1 was evaluated to evaluate the preliminary potential as a non-cationic and non-ferrous miRNA supply nanoplatform. Insignificant modifications have been noticed within the particle sizes of the GOA/miR-NC nanocomplex in an aqueous part over 14 days, indicating wonderful storage stability of the GOA/miR-NC nanocomplex (Fig. 1D). The dimensions of the nanocomplex remained unaltered at 200 nm after even a 100-fold dilution, suggesting a good dilution stability that’s extremely fascinating for intravenous administration (Fig. 1E). The salt and serum stability properties of this formulation are evidenced by the virtually fixed sizes at ~ 150 nm in water and at ~ 200 nm in a PBS answer regardless of additional addition of any totally different media, together with water, PBS, Opti-MEM, and DMEM (Fig. 1F).
Subsequent, the blood compatibility of the GOA/miR-NC nanocomplex was evaluated, which revealed insignificant OD worth modifications in fetal bovine serum (FBS) inside 5 days (Fig. 1G) with none prevalence of pink blood cell hemolysis (Fig. 1H) for the GOA/miR-NC nanocomplex. Extra importantly, the luminescent bands comparable to the GOA/miR-NC nanocomplex remained detectable on the gel even after 24 h of incubation with FBS (Fig. 1I) and was additional supported within the nuclease enzyme defend take a look at (Fig. S5). The outcomes strongly assist the improved colloidal stability of GOA/miR nanocomplex towards miRNA degradation over a comparatively prolonged length, which ensures the transfection of GOA/miR nanocomplex to most cancers cells in a serum-containing medium.
Preparation and characterization of GOA/miR nanocomplex. (A) Gel electrophoresis bands and (B) particle dimension of GOA/miR-NC nanocomplex at totally different molar ratios. (C) Consultant TEM photos of GOA/miR-NC nanocomplex. Scale bars: 200 nm. (D) The storage stability of GOA/miR-NC nanocomplex at room temperature for 14 days. (E) The particle dimension and PDI of GOA/miR-NC nanocomplex in several media. (F) Dilution stability of GOA/miR-NC nanocomplex in PBS. (G) Protecting results of GOA/miR-NC nanocomplex on stopping aggregation induced by serum proteins. (H) Hemolytic results of GOA/miR-NC nanocomplex. (I) Safety of miR-NC from FBS by GOA/miR nanocomplex. Knowledge characterize imply ± s.d. (n = 3)
Preparation and characterization of GOA/miR-363-5pi nanocomplex and GOA/miR-765i nanocomplex
The preparation of GOA/miR-363-5pi and GOA/miR-765i nanocomplexes was similar to the aforementioned protocols resulting in the GOA/miR-NC nanocomplex. Equally, the formation of stabilized GOA/miR-363-5pi and GOA/miR-765i nanocomplexes for full miR encapsulation occurred at a molar ratio of 250:1 and better ratios (Fig. 2A and B). The optimized GOA/miR-363-5pi and GOA/miR-765i nanocomplexes present imply particle sizes of roughly 200 nm (Fig. 2C) and adverse floor expenses of about − 20 mv (Fig. 2D). The DLS knowledge of three ready nanocomplexes at a molar ratio of 250:1 was summarized in Desk 1, which have been additional used for subsequent in vitro and in vivo evaluations. The profitable meeting of nanocomplexes with obvious adverse floor expenses confirms substantial miRNA encapsulation by way of hydrogen bonding somewhat than electrostatic interactions.
Preparation and characterization of GOA/miR-363-5pi and GOA/miR-765i nanocomplex. (A) The gel electrophoresis bands of GOA/miR-363-5pi and GOA/miR-765i nanocomplex at varied molar ratios. (B) The dimensions of the GOA/miR-NC, GOA/miR-363-5pi and GOA/miR-765i nanocomplex. (C) The particle dimension and (D) floor potential of GOA/miR-NC, GOA/miR-363-5pi and GOA/miR-765i nanocomplex
Mobile uptake of GOA/miR nanocomplex
Environment friendly mobile internalization is a prerequisite for profitable miRNA supply for particular gene silences. The mobile uptake of GOA/miR nanocomplexes was thus evaluated by way of movement cytometry and fluorescence microscopy. After therapy with GOA/miR nanocomplexes for 8 h, the imply fluorescence depth in Bel-7402 cells was 1.2 and three.4 -fold greater than these in HepG2 and SMCC-7721 cells, respectively (Fig. 3A), subsequently Bel-7402 cell line was chosen as the standard HCC cell mannequin for subsequent experiments (Fig. 3B). Notice that GOA/FAM-miR-NC nanocomplexes present fairly totally different uptake behaviors in three totally different HCC cell traces seemingly as a result of inherent properties of various HCC cell traces [24,25,26,27]. Sometimes, the compromised mobile uptake effectivity of GOA/FAM-miR-NC nanocomplexes in SMMC7721 and HepG2 cell traces signifies the slower uptake charges in these two cell traces in comparison with that in Bel-7402 cells.
Subsequent, the endocytosis of GOA/miR nanocomplexes in Bel-7402 cells was immediately visualized utilizing fluorescence microscopy. The inexperienced fluorescence depth of FAM within the GOA/FAM-miR group was considerably better than that noticed within the management group, indicating efficient miR transportation into the cells (Fig. 3C).
The lysosomal escape means of GOA/miR nanocomplex was additional investigated. Many of the inexperienced fluorescence (lysosome stained with Lyso-Tracker Inexperienced) and pink fluorescence (nanocomplexes labelled with Cy5) co-localized at 6 h, indicating that the nanocomplexes bear an intracellular lysosome pathway after endocytosis. The pink fluorescence of GOA/Cy5-miR nanocomplexes noticed within the cytoplasm elevated considerably after 8 h and 10 h of incubation, suggesting that the time-dependent lysosomal escape of GOA/miR nanocomplex happens primarily after 8 h (Fig. 3D). Taken collectively, the GOA/miR nanocomplex is an environment friendly miRNA supply vector for the Bel-7402 cell line-based HCC mannequin.
Mobile uptake of GOA/miR nanocomplex. (A) Circulation cytometry quantification of GOA/FAM-miR uptake after 8 h by three HCC cell traces, together with HepG2, SMCC-721, and Bel-7402 cells. Knowledge characterize imply ± s.d. (n = 3). *p < 0.05, ns: not vital. (B) The consultant photos of movement cytometry for GOA/FAM-miR in Bel-7402 cells. (C) Fluorescence microscopy photos of Bel-7402 cells incubated with free FAM-miR-NC, Lipo/FAM-miR-NC, and GOA/ FAM-miR-NC for 8 h. miRNA focus: 100 nM. (D) Confocal imaging of co-localization (yellow) of GOA/miR nanocomplex (Cy5-miR-NC, pink) with lysosomes (stained with Lyso-Tracker Inexperienced, inexperienced) in Bel-7402 cells. DAPI (blue) was used for staining the nucleus. Scale bars, 10 μm
In vitro antitumor results
To analyze the synergistic antitumor results of GOA/miR nanocomplex on Bel-7402 cells at totally different incubation durations, the inhibitory results of GOA/miR-363-5pi nanocomplex and GOA/miR-765i nanocomplex have been evaluated by MTT assays. In distinction to the insignificant inhibitory impact of free miRNA inhibitor on the expansion of tumor cells, the optimistic Lipo group did exhibit time-dependent cytotoxicity as a result of profitable supply of miR-363-5pi and miR-765i. Extra importantly, the nano-formulations incorporating GOA micelle parts demonstrated substantial cell inhibition at 24, 48, and 72 h of incubation in comparison with free gemcitabine, indicating enhanced antitumor results of gemcitabine facilitated by the supply programs (p < 0.001, Fig. 4A). Considerably decreased cell viabilities have been recorded within the GOA/miR-363-5pi (20.3%) and GOA/miR-765i nanocomplexes (15.5%), a lot decrease than these of GOA micelles (34.3%) and the GOA/miR-NC nanocomplex (34.3%) after an similar incubation interval of 48 h (p < 0.01). The decreased values of cell viability grew to become even better at a protracted incubation length of 72 h (p < 0.001), strongly supporting that miR-363-5pi and miR-765i contribute to the improved inhibitory impact of the nanocomplexes.
To offer further proof on the anti-tumor properties of the nanocomplexes, an EdU incorporation assay was carried out on Bel-7402 cells over 48 h. The group handled with GOA/miR-363-5pi or GOA/miR-765i nanocomplexes exhibited a considerably decreased inexperienced fluorescence depth of EdU in comparison with these of the opposite teams (Fig. 4B), indicating a noteworthy inhibition of HCC cell proliferation induced by the GOA/miR-363-5pi or GOA/miR-765i nanocomplexes. Curiously, the transfection of miR-363-5pi and miR-765i confirmed comparable cell antiproliferation charges in each the MTT assay and the EdU experiment no matter utilizing Lipo or GOA, which suggests an equal antitumor impact by these two miRNAs.
In vitro antitumor results of GOA/miR-363-5pi and GOA/miR-765i nanocomplex. (A) Cell viability of Bel-7402 cells incubated with totally different formulations after 24 h, 48 h, and 72 h. Knowledge characterize imply ± s.d. (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, ns: not vital. (B) Consultant EdU fluorescent photos on Bel-7402 cells after therapy with totally different formulations. Scale bar, 50 μm
In vitro antitumor mechanism
To confirm the scientific speculation that GOA/miR nanocomplex induces ferroptosis by upregulating LHPP and subsequently downregulating the PI3K signaling pathway, western blotting experiments have been used to analyze the alterations in proteins related to LHPP and the PI3K pathway in tumor cells. In comparison with these of the conventional liver cell line L02, the decreased expression of LHPP was noticed in three HCC cell traces, together with HepG2, SMCC-7721, and Bel-7402 cells (Fig. 5B), which emphasizes the vital function of miRNA supply in HCC remedy. Following 48 h of therapy with GOA/miR-363-5pi or GOA/miR-765i nanocomplexes, LHPP protein expression in Bel-7402 cells grew to become greater than these of the opposite teams (Fig. 5C), suggesting the profitable transfection of miR-363-5pi or miR-765i for LHPP overexpression. Curiously, the GOA/miR-765i nanocomplex demonstrated an LHPP protein expression degree just like that of the GOA/miR-363-5pi nanocomplex. Once more, the outcomes indicate comparable ferroptosis results of the 2 miRNAs.
LHPP can dephosphorylate the Histidine and seems to be a possible regulator of the PI3K/Akt pathway as a histidine phosphatase [15]. To elucidate the function of LHPP within the PI3K/AKT pathway, we evaluated the expression of key proteins on this pathway modulation and the respective phosphorylation ranges. Compared to these of the management teams, the protein ranges concerned within the PI3K/AKT pathway remained unaltered following therapy with free gemcitabine, GOA, and GOA/miR-NC, which means that neither gemcitabine nor GOA may make any alterations to the PI3K/AKT pathway (Fig. 5D). Nevertheless, in contrast with these of the management group, considerably decreased ranges of p-PI3K and p-Akt have been noticed upon transfection with GOA/miR-363-5pi or GOA/miR-765i nanocomplexes, whereas the PI3K or Akt ranges remained according to values just like that of the optimistic management Lipo/miR group. The deactivation of the phosphorylated PI3K/Akt pathway may very well be seemingly attributed to the profitable supply of miR-363-5pi or miR-765i for elevated LHPP expression.
Subsequently, we evaluated whether or not the inactivation of the PI3K/Akt pathway may set off ferroptosis in HCC. Glutathione peroxidase4 (GPX4) expression and lipid peroxide ranges have been employed as typical markers to verify ferroptosis prevalence. GPX4, with the flexibility to scavenge membrane lipid hydrogen peroxide merchandise, has been recognized because the central regulator of ferroptosis [28]. Compared to the management group, lipo-mediated miR transfection considerably downregulated GPX4 protein expression, whereas Gem, GOA micelles, and GOA/miR-NC nanocomplex confirmed indiscernible results on GPX4 expression, suggesting miR may downregulate the GPX4 expression no matter gemcitabine participation (Fig. 5E). Moreover, the grey worth intensities of the GPX4 bands within the GOA/miR-363-5pi and GOA/miR-765i nanocomplex teams have been decrease than these of the GOA micelles and GOA/miR-NC nanocomplex teams, indicating profitable miR supply by the nanocomplexes for ferroptosis induction in HCC cells.
With decreased GPX4 expression, accumulation of lipid peroxides happens, which ends up in cell membrane harm and ferroptosis prevalence. Additional quantification of lipid peroxide accumulation was carried out by BODIPY C11 staining coupled with movement cytometry evaluation in Bel-7402 cells after 48 h of therapy with GOA/miR-363-5pi or GOA/miR-765i nanocomplexes. Compared to these of the management and free miR teams, elevated ranges of lipid peroxides have been seen in each Gem and GOA teams, which may doubtlessly be attributed to an imbalance within the intracellular redox equilibrium brought on by cytotoxicity of gemcitabine (Fig. 5F). Moreover, the GOA/miR-363-5pi or GOA/miR-765i nanocomplexes exhibited FCM intensities 1.5-fold better than that of the GOA group, indicating the miR supply can successfully upregulate the lipid peroxide ranges for additional triggering ferroptosis in most cancers cells in flip.
Lastly, to verify the prevalence of ferroptosis in Bel-7402 cells, a rescue experiment with ferrostatin-1 (Fer-1), a ferroptosis inhibitor, was carried out after GOA/miR-363-5pi or GOA/miR-765i nanocomplex therapy. At 48 h and 72 h of incubation, the co-administration of Gem, GOA micelles, and GOA/miR-NC nanocomplex with Fer-1 didn’t improve cell viability relative to Fer-1 alone, indicating gemcitabine alone couldn’t set off ferroptosis. Conversely, Fer-1 reversed the decreased viability of Bel-7402 cells handled with both GOA/miR-363-5pi or GOA/miR-765i nanocomplexes (p < 0.001, Fig. 5G), suggesting that the cell demise mode induced by miR-363-5pi or miR-765i was certainly ferroptosis. It’s noteworthy that the transfection of miR-765i induced ferroptosis results that have been primarily similar to these of miR-363-5pi, which can be defined by the comparable upregulated LHPP protein expression ranges by each miRNAs.
Collectively, the potent anti-HCC results of GOA/miR-363-5pi and GOA/miR-765i nanocomplexes have been primarily attributed to the mixture of gemcitabine-induced chemotherapy and miR-mediated ferroptosis, which upregulates LHPP expression and inactivates the PI3K/Akt pathway. We, subsequently, draw a conclusion that GOA/miR-363-5pi and GOA/miR-765i nanocomplexes function ferroptosis inducers within the absence of ferrous nanoparticles.
In vitro antitumor mechanism. (A) Schematic illustration of the ferroptosis-induced mechanism after GOA/miR-363-5pi or GOA/miR-765i nanocomplex therapy. (B) LHPP expression in three HCC cell traces. (C) The expression of LHPP in Bel-7402 cells after therapy with totally different part formulations. (D) The protein ranges of whole PI3K (PI3K), phosphorylated PI3K (p-PI3K), whole AKT (AKT), and phosphorylated AKT (p-AKT) have been decided by Western blotting. (E) Expression of GPX4 in Bel-7402 cells after 48-hour therapy with totally different formulations. (F) Intracellular lipid accumulation was assessed by movement cytometry following staining with BODIPY in Bel-7402 cells after therapy with totally different formulations. (G) Ferroptosis inhibitors ferrostatin-1 (*) rescue Bel-7402 cell demise induced by GOA/miR-363-5pi or GOA/miR-765i nanocomplex at 48 h and 72 h. Knowledge characterize imply ± s.d. (n = 3). **p < 0.01, ***p < 0.001, ns: not vital
In vivo anti-HCC results
Efficient tumor tissue accumulation is a prerequisite for anti-HCC results. Cy5.5 was used as a fluorescent dye to label the nanocomplexes by way of bodily encapsulation. The ensuing Cy-5.5-labelled nanocomplexes, GOA/miR-Cy5.5 underwent tail vein injection in BALB/c nude mice bearing Bel-7402 tumor xenografts, and the fluorescence distributions in main organs and tumors have been subsequently visualized in a FUSION FX imaging system (VilberLourmat, France) (Fig. S6A). The fluorescence depth of Cy5.5 reached a peak worth at about 8 h publish injection and fully disappeared after 24 h within the tumor web site (Fig. S6B), indicating that the GOA/miR-Cy5.5 nanocomplex may successfully accumulate within the tumor via passive concentrating on by way of an EPR impact. Related time-dependent fluorescence depth modifications have been recorded within the main organs (Fig. S6C). The biodistribution knowledge reveal that GOA/miR nanocomplex had the potential to focus on HCC with out damaging regular organs.
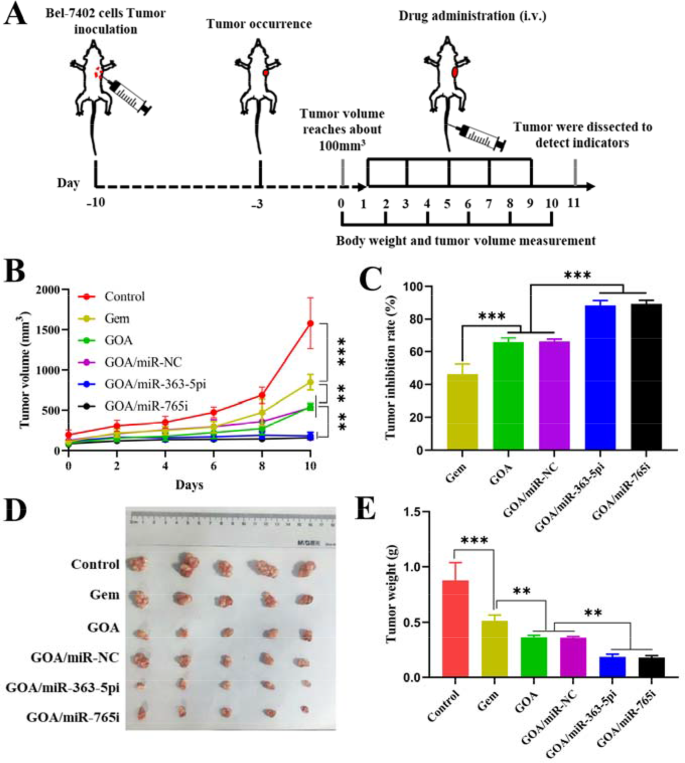
Inspired by GOA/miR nanocomplex biodistribution outcomes, the antitumor results of GOA/miR nanocomplex have been additional evaluated within the BALB/c nude mice bearing Bel-7402 tumor xenografts. Six teams of tumor-bearing mice have been intravenously injected with saline, Gem, GOA micelles, GOA/miR-NC nanocomplex, GOA/miR-363-5pi nanocomplex, and GOA/miR-765i nanocomplex at 48-hour intervals for a complete of 5 administrations (Fig. 6A). In comparison with the saline group, each free gemcitabine and all GOA-based formulations exhibited vital inhibitory results on tumor progress (Fig. 6B). In distinction to the GOA/miR-NC nanocomplex, each GOA/miR-363-5pi and GOA/miR-765i nanocomplexes exhibited virtually flattened tumor progress profiles (p < 0.001). Mice handled with GOA/miR-363-5pi and GOA/miR-765i nanocomplexes confirmed 88% and 89% regression in tumor volumes, respectively, which have been considerably greater than these of Gem (46%), GOA micelles (66%), and GOA/miR-NC nanocomplex (66%) teams (Fig. 6C). The superb therapy effectivity of each nanocomplexes was additional supported by the pictures of ex vivo tumor tissues (Fig. 6D) and the common weight of the tumor tissues (Fig. 6E) extracted from the mice sacrificed on the eleventh day. According to the in vitro findings, miR-765i confirmed in vivo anti-tumor efficacy virtually similar to that of miR-363-5p.
In vivo analysis of the therapeutic results of GOA/miR-363-5pi and GOA/miR-765i nanocomplexes in HCC therapy. (A) Experiment schedule of anti-tumor examine in Bel-7402-tumor-bearing BALB/c mice. (miRNA dose: 1 mg/kg, administered intravenously each 2 days for a complete of 5 doses). (B) Tumor quantity and (C) tumor inhibition fee of Bel-7402 tumor-bearing BALB/c mice handled with totally different formulations. (D) Images and (E) tumor weight of ex vivo tumor tissues on the finish of therapy. Knowledge characterize imply ± s.d. (n = 5). **p < 0.01, ***p < 0.001
In vivo LHPP expression and ferroptosis induction
An in-depth investigation of the in vivo antitumor mechanism of GOA/miR-363-5pi and GOA/miR-765i nanocomplexes was lastly performed. In keeping with the in vitro outcomes, the immunohistochemistry photos of tumor tissues indicated vital upregulation of LHPP expression following therapy with both the GOA/miR-363-5pi or GOA/miR-765i nanocomplexes, in comparison with these of Gem, GOA micelles, and GOA/miR-NC nanocomplex (Fig. 7A). This statement was additional supported by the upper common OD worth of LHPP, as decided by the semi-quantitative immunohistochemical evaluation (p < 0.001, Fig. 7B). The elevated LHPP expression primarily resulted from the profitable supply of miR-363-5pi and miR-765i by way of the GOA/miR nanocomplex.
The precise ferroptosis markers, together with the extent of GPX4 and lipid peroxidation in tumor tissues, have been assessed utilizing immunohistochemistry and movement cytometry, respectively. In distinction to the noticed LHPP expression pattern, a decreased GPX4 expression was seen in tumor tissues handled with the GOA/miR-363-5pi and GOA/miR-765i nanocomplexes in comparison with these of the opposite teams (Fig. 7A), which was additional confirmed by a semi-quantitative evaluation of fluorescence depth (Fig. 7C). Moreover, the lipid peroxidation ranges in mice tumor tissue handled with GOA/miR-363-5pi and GOA/miR-765i nanocomplexes have been roughly ~ 1.37 and 1.41-fold greater than that of the management group, respectively (Fig. 7D). These values have been considerably better than these noticed of the mice tumor tissue handled with Gem (~ 1.01-fold), GOA micelles (~ 1.19-fold), and GOA/miR-NC nanocomplex (~ 1.21-fold) (p < 0.001). According to the in vitro findings, miR-765i had an upregulation impact on LHPP expression in vivo just like that of miR-363-5p, suggesting the same potencies of the 2 miRNAs in ferroptosis induction. Collectively, the aforementioned organic proof helps GOA/miR-363-5pi or GOA/miR-765i nanocomplexes-mediated efficient ferroptosis by way of LHPP protein expression upregulation.
The improved therapeutic efficacy of the GOA/miR-363-5pi and GOA/miR-765i nanocomplexes, in comparison with these of the opposite teams, in all fairness attributed to the profitable co-delivery of miR-363-5pi or miR-765i with Gem. Subsequently, the supply system performs an vital function in mediating combinatory remedy by way of simultaneous LHPP-triggered ferroptosis and GOA-induced chemotherapy, outperforming the efficacy of chemotherapy alone, for enhanced therapy of HCC.
In vivo ferroptosis induced by the therapy of GOA/miR-363-5pi and GOA/miR-765i nanocomplex. (A) Immunohistochemistry assay of LHPP and GPX4 expression in tumor tissues from Bel-7402-tumor-bearing BALB/c mice. (B) Semiquantitative evaluation of immunohistochemical AOD for LHPP and (C) GPX4 by utilizing Picture J software program. (D) Relative quantification of peroxidized lipids collected within the tumor tissues was analyzed via movement cytometry. Knowledge characterize imply ± s.d. (n = 5). ***p < 0.001
In vivo security evaluation
The in vivo biosafety of nano-inducers, akin to GOA/miR-363-5pi and GOA/miR-765i nanocomplexes, is of paramount concern. Physique weights have been measured each 2 days, as a decreased physique weight profile was an apparent indication of extreme toxicity. In the entire experimental interval, insignificant modifications in mice weights have been noticed after therapy with the GOA-based nanocomplexes whereas substantial weight losses have been seen after 4 days of Gem therapy, highlighting the superior biosafety of the nanocomplex (Fig. 8A).
Attributable to Gem’s tendency to induce hemotoxic unwanted side effects in clinics, blood samples have been collected and analyzed from mice handled with totally different formulations for 11 days. No observable harm to pink or white blood cells was evident in any of the experimental teams (Fig. S7). Nevertheless, the variety of platelets was decreased within the Gem group, a well known poisonous facet impact in scientific settings. Notably, this decline was prevented by GOA-based formulations, seemingly as a result of exact drug supply of the nanocomplex for minimized accumulation on the non-target web site, and the noncationic properties of GOA-based formulations for decreased nanocomplex toxicity to cell membranes (Fig. 8B). General, the intravenous injection of GOA/miR-363-5pi and GOA/miR-765i nanocomplexes didn’t induce any detrimental results on blood cell counts.
Lastly, histological evaluation of tumor tissues and important organs, together with the guts, liver, and kidney, was carried out utilizing H&E staining. The key organs exhibited no evident pathological abnormalities. As anticipated, the tumor tissues of nude mice handled with the GOA/miR nanocomplex displayed extra apparent cytoplasmic disappearance, karyopyknotic, and apoptosis (indicated as arrows) in comparison with these of the opposite teams (Fig. 8C). No vital distinction in serum inflammatory elements IL-6 and IFN-α was noticed between the all of teams, confirming that GOA/miR-363-5pi and GOA/miR-765i nanocomplexes didn’t result in a systemic inflammatory response (Fig. S8). Subsequently, the applying of GOA/miR-363-5pi and GOA/miR-765i nanocomplexes demonstrated vital antitumor efficacy whereas minimizing systematic toxicity on regular organs and tissues for enhanced potential suitability for in vivo functions.
In vivo security evaluation of various formulations in BALB/c nude mice bearing Bel-7402 xenograft mannequin. (A) Physique weight of Bel-7402-tumor-bearing BALB/c nude mice handled with totally different formulations. (B) The degrees of platelet within the serum after therapy on the eleventh day. (C) H&E staining of the tumor, coronary heart, liver, and kidney (scale bars: 100 μm). Knowledge characterize imply ± s.d. (n = 5). **p < 0.01, ***p < 0.001
Potential for immune activation
At the moment, the induction of immunogenic cell demise (ICD) by combining a number of therapies is an extremely promising method for advancing most cancers remedy. To evaluate the immune activation potential of the GOA/miR-363-5pi nanocomplex, the discharge of damage-associated molecular patterns (DAMPs), which function a signature for immunogenic cell demise (ICD), was investigated in Bel-7402 cells handled with nanocomplex. The strongest calreticulin (CRT) fluorescence, in addition to the bottom HMGB1 fluorescence, may very well be clearly noticed after GOA/miR-363-5pi and GOA/miR-765i nanocomplexes therapies (Fig. 9A), which demonstrated that the synergistic technique of chemotherapy and ferroptosis triggered by GOA/miR-363-5pi nanocomplex stimulated efficient CRT publicity and HMGB1 launch. As well as, extracellular HMGB1 (Fig. 9B) and ATP (Fig. 9C) concentrations have been considerably enhanced within the GOA/miR-363-5pi and GOA/miR-765i nanocomplexes teams than these within the Gem, GOA, and GOA/miR-NC nanocomplex teams (p < 0.001) detected by Elisa equipment, which demonstrated that GOA/miR-363-5pi nanocomplex stimulated HMGB1 and ATP launch from Bel-7402 cells into the extracellularly. Thus, the GOA/miR-363-5pi nanocomplex possesses the capability to induce ICD via the stimulation of DAMPs launch, which holds the potential to activate anti-tumor immune response.
Evaluation of immunogenic cell demise of GOA/miR nanocomplex. (A) The characterize picture of CRT (scale bars: 200 μm) and HMGB1 (scale bars: 100 μm) detected by fluorescence microscope in Bel-7402 cells after therapy by GOA/miR-363-5pi nanocomplex and GOA/miR-765i nanocomplex. Concentrations of (B) HMGB1 and (C) ATP in cell tradition medium after therapy with every part. Knowledge characterize imply ± s.d. (n = 3). **p < 0.01, ***p < 0.001, ns: not vital