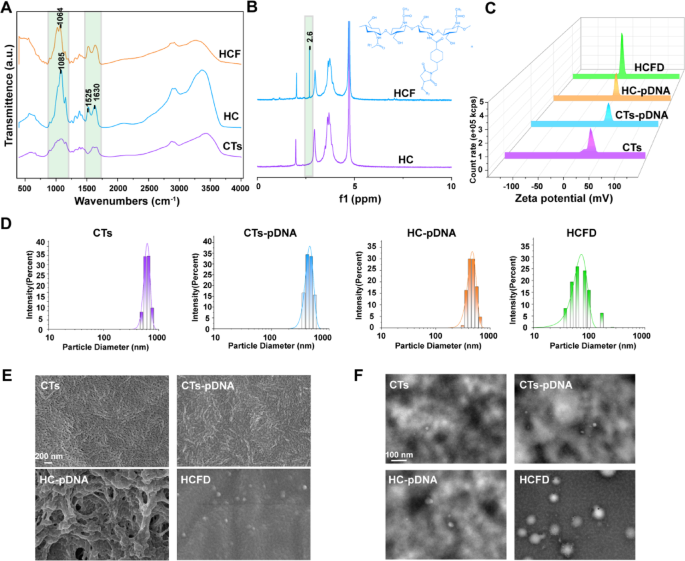
Preparation and characterization of HCFD nanoparticles
To substantiate that FAP1 and His have been efficiently hooked up to CTs, we carried out infrared spectroscopy on CTs, HC (His-CTs), and HCF (His-CTs-FAP1 peptide). In comparison with CTs, HC reveals an enhanced absorption peak at 1085 cm−1 in its infrared spectrum. This peak could also be related to vibrations of glycosyl or His residues, indicating the formation of latest bonds [26,27,28]. The attribute peak shift at 1525 cm−1 and the improved absorption peak at 1630 cm−1 correspond to the amide I band (C = O stretching vibration), which usually signifies the formation or strengthening of amide bonds. This phenomenon is often noticed throughout peptide-polymer conjugation and serves as a chemical signature for the binding of proteins or peptides to carriers akin to CTs. The noticed enhancement could originate from the covalent linkage between His peptides and CTs (Fig. 1A) [29]. In comparison with HC, the infrared spectrum of HCF reveals diminished depth on the attribute peak at 1064 cm⁻¹. This discount could also be attributed to the formation of an intermolecular hydrogen bond between the guanidino group (-NH-C(= NH)NH₂) of the arginine residue within the FAP1 peptide and the phenolic hydroxyl group (-OH) of tyrosine (Fig. 1A) [30]. We additional validated this impact utilizing nuclear magnetic resonance (NMR) spectroscopy. The outcomes revealed that in comparison with the HC group, the NMR spectra of the HCF group exhibited a brand new peak at 2.6 ppm, doubtless originating from the introduction of methylene or methyl teams from the FAP1 peptide (Fig. 1B) [31]. These findings urged that FAP1 and His have been efficiently conjugated to CTs.
We constructed the plasmid pGPU6/GFP/Neo MCP-1-shRNA to stably knock out MCP-1. To confirm whether or not pDNA was efficiently loaded onto HCF nanoparticles, we measured the zeta potential of CTs, CTs-pDNA, HC-pDNA, and HCF-pDNA (HCFD). In contrast to CTs, CTs-pDNA, HC-pDNA, and HCFD exhibited a detrimental potential (Fig. 1C), according to the Part Picture (Fig. S2). We additional measured the particle dimension adjustments of CTs, HC, and HCF, and located that that of HCFD nanoparticles primarily ranged from 50 to 100 nm (Fig. 1D). The morphological alterations of pDNA following binding to CTs, HC, and HCF have been investigated utilizing scanning electron microscopy (SEM) and transmission electron microscopy (TEM). In distinction to CTs, CTs–pDNA, and HC–pDNA, HCFD produced spherical particles that have been primarily between 60 and 90 nm in diameter, which is in step with the particle dimension vary that DLS detected (Fig. 1E, F). The above outcomes indicated that in comparison with CTs and HC, HCF is extra readily able to forming spherical carriers with pDNA. The primary particle dimension of those spherical carriers is lower than 100 nanometers, which helps them enter cells extra successfully and carry out their capabilities.
Preparation and characterization of HCFD nanoparticles. (A) Infrared spectroscopy evaluation of CTs, HC, and HCF. (B) Nuclear magnetic resonance hydrogen spectroscopy evaluation of HC and HCF. (C) Zeta potential measurement of CTs, CTs–pDNA, HC–pDNA, and HCFD. (D) Particle dimension distribution of CTs, CTs–pDNA, HC–pDNA, and HCFD. (E) SEM evaluation of CTs, C–pDNA, HC–pDNA, and HCFD. (F) TEM evaluation of CTs, C–pDNA, HC–pDNA, and HCFD
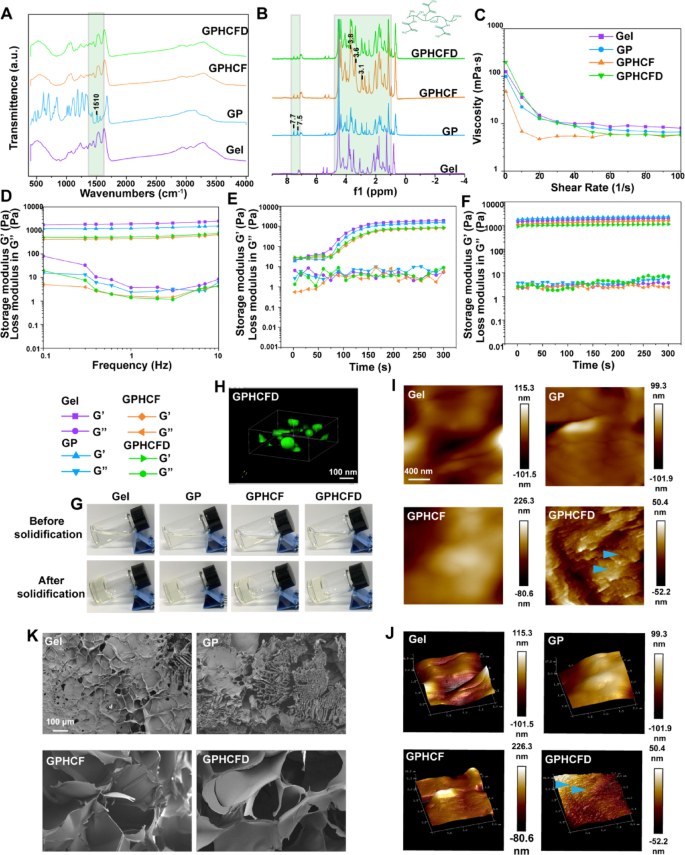
Synthesis and bodily and chemical characterization of the GPHCFD hydrogel
We carried out infrared spectroscopy on Gelma (Gel), GP, GPHCF, and GPHCFD to confirm the profitable formation of the GPHCFD hydrogel. Within the mid-infrared area, the attribute peak at 1510 cm⁻¹ corresponded to the vibration of the benzene ring skeleton, indicating the profitable incorporation of benzene-containing parts into the hydrogel [32] (Fig. 2A). The infrared spectra of GPHCF and GPHCFD confirmed no adjustments as compared with that of GP, which was attributable to the extreme variety of teams hooked up to GP in GPHCF and GPHCFD (Fig. 2A) [33].
In distinction to GelMA, GP confirmed extra peaks at 7.7 ppm and seven.5 ppm, which correspond to the ortho and para protons of the PBA benzene ring, respectively, in keeping with a nuclear magnetic resonance hydrogen spectroscopy research [34]. The shift at 7.5 ppm signifies enhanced polarity of the B-O bond, resulting in a conjugation impact, which is according to the anticipated formation of a borate ester bond (B-OR) with PBA (Fig. 2B). This displays the formation strategy of dynamic covalent bonds. On the similar time, the improved peaks at 3.1, 3.6, and three.8 ppm within the H3-H6 proton area of the CTs sugar ring could point out intermolecular interactions, akin to hydrophobic results, according to the mechanism of chitosan binding to FAP1 (Fig. 2B) [34]. Moreover, the high-molecular-weight matrix background obscures the pDNA sign; subsequently, there is no such thing as a discernible distinction between the GPHCFD and GPHCF spectra [35]. The aforementioned findings urged the profitable synthesis of GPHCFD.
We carried out rheological characterization of Gel, GP, GPHCF, and GPHCFD hydrogels. Shear charge scanning assessments confirmed that throughout the γ̇ = 1–10 s−1 vary (comparable to injection charges of 0.1-1 mL/min), GPHCF and GPHCFD exhibited important shear thinning conduct, with viscosity (η) maintained at 7–20 mPa·s (Fig. 2C), confirming their injectability [36]. The event of a secure gel community was demonstrated by oscillation time scans. It confirmed that GPHCFD’s storage modulus (G’) remained increased than its loss modulus (G’’) (Fig. 2D). The curing time of GPHCFD hydrogel, influenced by the added CTs, ranged from 50 to 80 seconds (Fig. 2E, G). Frequency scanning revealed that the G’ worth of GPHCFD hydrogel is beneath 1000 Pa (Fig. 2F), approaching the mechanical properties of soppy tissues (e.g., adipose tissue ~ 300 Pa), which is advantageous for subsequent biomedical functions [37].
Fluorescence confocal microscopy was used to generate 3D photographs of fluorescently labeled HCFD nanoparticles in GPHCFD hydrogels, demonstrating that HCFD nanoparticles had efficiently hooked up to the GPHCFD hydrogels (Fig. 2H). Atomic pressure microscopy (AFM) evaluation of 2D and 3D photographs of the Gel, GP, GPHCF, and GPHCFD hydrogels revealed that solely GPHCFD exhibited a definite granular texture. GPHCFD contained a lot of HCFD nanoparticles (Fig. 2I, J), with particle sizes starting from 50 to 90 nm (Fig. S3). SEM evaluation was used to look at the morphology of the Gel, GP, GPHCF, and GPHCFD hydrogels. Including a pore-forming agent throughout GP synthesis and linking HCF and HCFD resulted in GPHCF and GPHCFD hydrogels exhibiting bigger pores (Fig. 2Okay). The synthesized GPHCFD hydrogel facilitated the efficient launch of HCFD nanoparticles. These outcomes urged the profitable meeting of GPHCFD hydrogels (loaded HCFD nanoparticles). These outcomes additionally indicated that the GPHCFD hydrogels have been appropriate for subsequent nanoparticle supply and cell experiments.
Synthesis and physicochemical characterization of the GPHCFD hydrogel. (A) Infrared spectroscopy evaluation of the Gel, GP, GPHCF, and GPHCFD hydrogels. (B) Nuclear magnetic resonance hydrogen spectroscopy evaluation of the Gel, GP, GPHCF, and GPHCFD hydrogels. (C) Viscosity measurements of the Gel, GP, GPHCF, and GPHCFD hydrogels. (D) Frequency sweep evaluation of the Gel, GP, GPHCF, and GPHCFD hydrogels. (E) Photocuring time evaluation of the Gel, GP, GPHCF, and GPHCFD hydrogels. (F) Self-healing evaluation of the Gel, GP, GPHCF, and GPHCFD hydrogels. (G) Typical photographs of the Gel, GP, GPHCF, and GPHCFD hydrogels earlier than and after curing. (H) Fluorescence confocal microscopy of nanoparticles in GPHCFD hydrogel. (I, J) AFM evaluation of the Gel, GP, GPHCF, and GPHCFD liquid hydrogels. (Okay) SEM photographs of the Gel, GP, GPHCF, and GPHCFD hydrogels
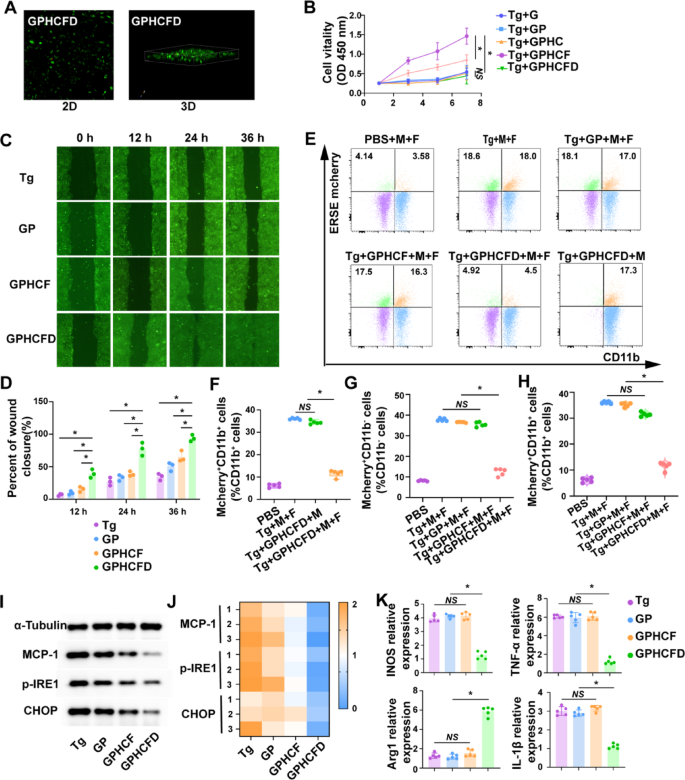
Organic perform testing of ERS-responsive GPHCFD hydrogels
Fluorescence confocal imaging of fibroblasts cultured on GPHCFD hydrogels (2D/3D) confirmed multidirectional progress (inexperienced) and minimal cell demise (pink) (Fig. 3A), indicating good biocompatibility. The GPHCD hydrogel exhibited the strongest promotion of fibroblast cell proliferation when fibroblast cells have been induced to endure ERS in vitro utilizing Tg. Solely GPHCFD and GPHCF hydrogels demonstrated a big promotional impact on fibroblast proliferation. Subsequently, we chosen GPHCD and GPHCF because the experimental teams and GP because the management group for the next experiments (Fig. 3B). These outcomes counsel that extreme ERS can activate GPHCFD hydrogel launch plasmid, knock down the MCP-1 gene in fibroblasts, and inhibit the expression of MCP-1. Induce the ERS in fibroblasts utilizing Tg concentrations of 0 mM, 10 mM, 50 mM, and 100 mM. Utilizing stream cytometry, assess the fluorescence depth of cells transfected with a GFP-labeled plasmid. The GFP fluorescence depth was considerably elevated within the 50 mM and 100 mM Tg induction teams, with the best GFP fluorescence depth noticed within the 100 mM Tg induction group. This indicated that as ERS elevated in fibroblasts, plasmid transfection effectivity was considerably enhanced (Fig. S4). Among the many 4 teams of Tg-induced fibroblasts that underwent ERS–the non-hydrogel group, GP group, GPHCF group, and GPHCFD hydrogel group–the GPHCFD hydrogel group had the quickest migration pace of fibroblasts (Fig. 3C, D).
Fibroblast (F) and macrophage (M) cell strains expressing ESRE mCherry have been established. When extreme ERS happens, each fibroblasts and macrophages present optimistic expression of ESRE mCherry. Within the coculture system, CD11b+ cells represented macrophages, and CD11b− cells represented fibroblasts. The next teams have been analyzed: PBS with out Tg induction, Tg-induced fibroblasts and macrophages, Tg-induced macrophages, GP hydrogel-treated Tg-induced fibroblasts and macrophages, GPHCF hydrogel-treated Tg-induced fibroblasts and macrophages, and GPHCFD hydrogel-treated Tg-induced fibroblasts and macrophages. The proportion of ESRE mCherry+CD11b+ cells was considerably increased in Tg-induced fibroblasts and macrophages and in Tg-induced macrophages, whereas the proportion of ERSE mCherry+CD11b+ cells was very low within the non-Tg-induced PBS group and in hydrogel-treated Tg-induced fibroblasts and macrophages. This discovering indicated that the GPHCFD hydrogel was ERS-responsive and will solely exert its ERS-inhibitory perform within the presence of fibroblasts. The GPHCFD hydrogel focused fibroblasts and inhibited ERS in each fibroblasts and macrophages (Fig. 3E, F). The proportion of ERSE mcherry+CD11b− cells was excessive within the Tg-induced fibroblast and macrophage teams, the GP hydrogel-treated Tg-induced fibroblast and macrophage teams, and the GPHCF hydrogel-treated Tg-induced fibroblast and macrophage teams. In distinction, the proportion of ERSE mcherry+CD11b− cells was low within the non-Tg-induced PBS group and the GPHCDFD hydrogel-treated Tg-induced fibroblast and macrophage group (Fig. 3E, G). Whereas the non-Tg-induced PBS group and the GPHCFD hydrogel-treated Tg-induced fibroblast and macrophage teams confirmed a low proportion of mcherry+CD11b+ cells, the Tg-induced fibroblast and macrophage group, the GP hydrogel-treated Tg-induced fibroblast and macrophage group, and the GPHCF hydrogel-treated Tg-induced fibroblast and macrophage group confirmed a excessive proportion of ERSE mcherry+CD11b+cells (Fig. 3E, H), suggesting that the ERS-responsive GPHCFD can concurrently inhibit ERS in fibroblasts and macrophages. Tg-induced fibroblasts and macrophages underwent ERS. A coculture system was established with two cell sorts divided into the no hydrogel, GP, GPHCF, and GPHCFD hydrogel-treated teams. Western blotting evaluation of fibroblasts within the coculture system revealed that the GPHCFD group exhibited the bottom expression ranges of MCP-1 and the ERS-specific molecules p-IRE1 and CHOP among the many 4 teams, confirming that the GPHCFD hydrogel can inhibit MCP-1 expression in fibroblasts and scale back fibroblast ERS ranges (Fig. 3I, J, S5). The GPHCFD group confirmed the best abundance of the M2-type attribute molecule Arg1 and the bottom expression of the M1-type attribute molecules TNF-α, iNOS, and IL-1β, in keeping with fluorescent quantitative PCR evaluation of macrophages within the coculture system. This urged that the GPHCFD hydrogel can forestall the promotion of M1 to M2 macrophage transformation (Fig. 3Okay). The above outcomes demonstrated that the GPHCFD hydrogel is an ERS-responsive hydrogel. By suppressing MCP-1 expression, the GPHCFD hydrogel may lower extreme ERS in fibroblasts and encourage fibroblast migration and proliferation. It may additionally lower ERS in macrophages and encourage the polarization of M1-type macrophages towards the M2 phenotype.
Organic perform testing of ERS-responsive GPHCFD hydrogels. (A) Fluorescence confocal detection of fibroblast proliferation within the GPHCFD hydrogels. Crimson: lifeless fibroblast; inexperienced: dwell fibroblast. (B) CCK8 detection of fibroblast proliferation within the Gel GP, GPHCF, and GPHCFD hydrogels induced by Tg on days 1, 3, 5, and seven, in that order. n = 3 per group. (C, D) Tg-induced fibroblasts produce extreme ERS; scratch assay of fibroblast migration within the no hydrogel, GP, GPHCF, and GPHCFD hydrogel-treated groups. n = 3 per staff. (E) Stream cytometry evaluation of the PBS group with out Tg induction, the Tg-induced fibroblast (F) and macrophage (M) group, the GP hydrogel-treated Tg-induced fibroblast and macrophage group, the GPHCF hydrogel-treated Tg-induced fibroblast and macrophage group, the GPHCFD hydrogel-treated Tg-induced fibroblast and macrophage group, and the GPHCFD hydrogel-treated Tg-induced macrophage group. (F) The proportion of mcherry+CD11b+ cells inside CD11b+ cells is statistically analyzed. n = 5 per staff. NS: non-significant, *P < 0.05. (G) The proportion of mcherry+CD11b− cells inside CD11b− cells is statistically analyzed. (H) The proportion of mcherry+CD11b+ cells inside CD11b+ cells is statistically analyzed. n = 5 per group. (I) Tg-induced 4 teams with out hydrogels; GP, GPHCF, and GPHCFD hydrogel-treated fibroblasts and macrophages underwent ERS. Western blotting was used to evaluate the expression ranges of MCP-1, p-IRE1, and CHOP in fibroblasts from the 4 teams. (J) The statistical evaluation was carried out through a heatmap. n = 3 per group. (Okay) Fluorescent quantitative PCR was used to measure the expression ranges of TNF-α, iNOS, IL-1β, and Arg1 in macrophages from the 4 teams. n = 5 per staff. NS: non-significant, *P < 0.05. The information is displayed as imply ± SD.
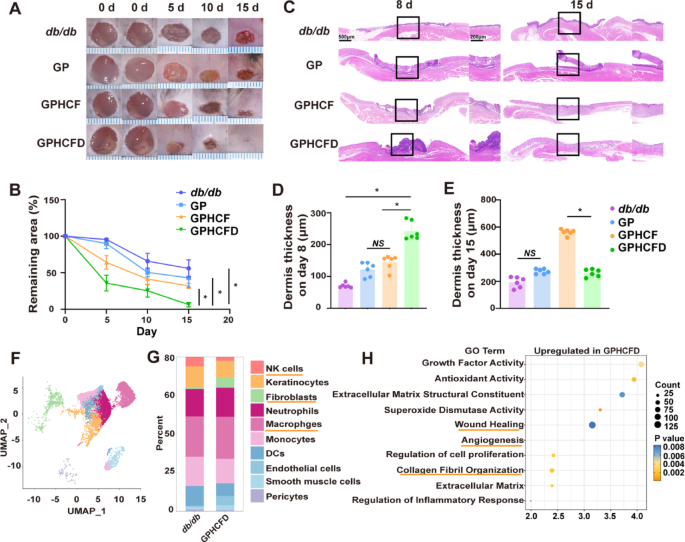
The GPHCFD hydrogel promoted diabetic wound therapeutic
We additional investigated the therapeutic results of the GPHCFD hydrogel on diabetic wounds. Three hydrogels, specifically, GP, GPHCF, and GPHCFD, have been used to deal with diabetic wounds, and the wound areas of the untreated, GP, GPHCF, and GPHCFD teams have been assessed. GPHCFD hydrogel and GPHCF hydrogel considerably promoted wound contraction as compared with that noticed within the untreated group, with the GPHCFD hydrogel displaying one of the best therapeutic impact (Fig. 4A, B). HE staining was used to evaluate adjustments within the dermis of the untreated, GP, GPHCF, and GPHCFD hydrogel teams. The GPHCFD and GPHCF hydrogels promoted dermal formation, with essentially the most important dermal thickening noticed within the GPHCFD hydrogel group on day 8 (Fig. 4C, D). By day 15, the dermis thickness within the GPHCFD hydrogel group had already begun to cut back (Fig. 4C, E), indicating that the dermis within the GPHCFD hydrogel group matured earliest. Moreover, on day 15, the GPHCFD hydrogel group exhibited extra dermal hair follicles than the opposite three teams (Fig. 4C).
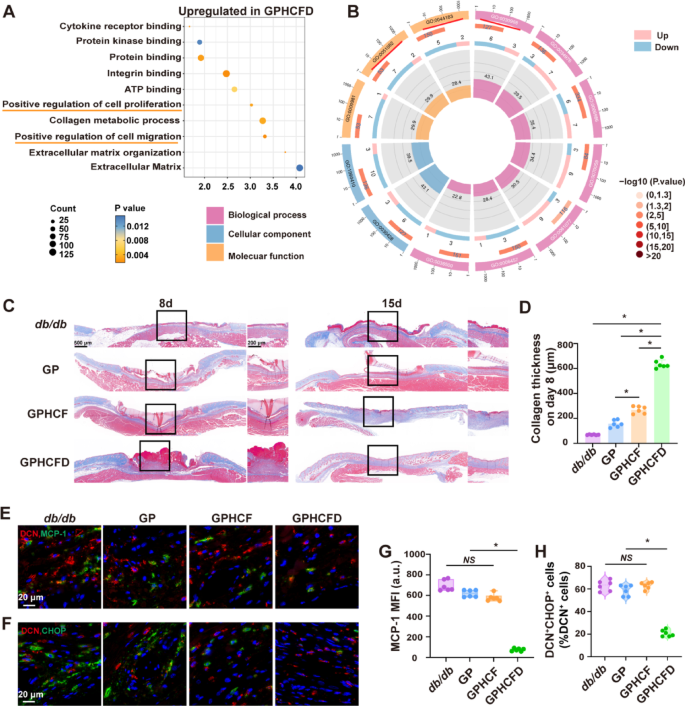
To discover the mechanism of motion of the GPHCFD hydrogel on wounds, we carried out single-cell sequencing on diabetic wound tissue with out remedy on day 3 and diabetic wound tissue handled with GPHCFD hydrogel. Wound cells have been categorised into 10 cell teams: fibroblasts, macrophages, neutrophils, NK cells, keratinocytes, monocytes, endothelial cells, pericytes, DCs, and clean muscle cells (Fig. 4F). The share of fibroblasts was a lot increased in GPHCFD hydrogel-treated diabetic wound tissue than in untreated diabetic wound tissue, however the proportion of NK cells was considerably decrease (Fig. 4G). GO enrichment evaluation associated to wound therapeutic confirmed that gene units associated to wound therapeutic, collagen fibril group, and angiogenesis have been considerably enriched within the GPHCFD hydrogel remedy group (Fig. 4H). The outcomes indicated that GPHCFD hydrogel promoted the therapeutic of diabetic wounds, doubtlessly by enhancing fibroblast proliferation and suppressing irregular pure killer cell proliferation.
The GPHCFD hydrogel facilitated diabetic wound therapeutic. (A) Typical photographs of diabetic wounds handled with no hydrogel, GP, GPHCF, and GPHCFD hydrogel at days 5, 10, and 15. n = 6 per group. (B) Statistical evaluation of the wound areas. (C) H&E stained photographs displaying wound therapeutic within the no hydrogel, GP, GPHCF, and GPHCFD hydrogel remedy teams for diabetic wounds. Scale bar, 1000 μm and 200 μm. (D, E) Statistical evaluation of dermal thickness within the 4 teams at days 8 and 15. n = 6 in every group. (F) UMAP plot evaluation of 10 cell clusters within the GPHCFD hydrogel-treated diabetic wound group. (G) Bar chart evaluation of the proportion of 10 cell clusters within the non-hydrogel-treated diabetic wound group and the GPHCFD hydrogel-treated diabetic wound group. (H) GO enrichment evaluation of wound healing-related gene units within the GPHCFD hydrogel-treated diabetic wound group. n = 3 per staff. The information is displayed as imply ± SD.
The GPHCFD hydrogel inhibited extreme ERS in fibroblasts by suppressing MCP-1 expression, thereby selling collagen formation
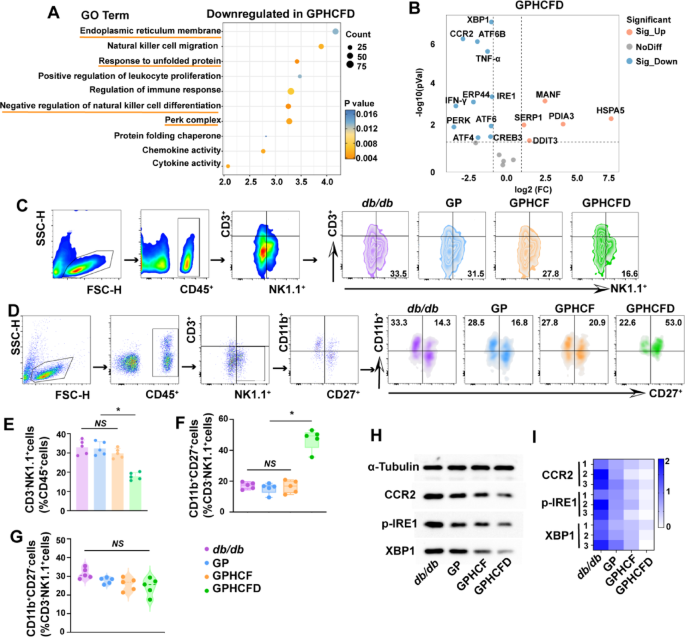
We analyzed the useful roles of native fibroblasts in diabetic wound tissue handled with the GPHCFD hydrogels utilizing single-cell sequencing information. GO enrichment evaluation confirmed that gene units associated to optimistic regulation of cell proliferation and migration have been considerably enriched in fibroblasts handled with the GPHCFD hydrogels (Fig. 5A). GO enrichment circle diagram evaluation confirmed that in diabetic wound tissue, native fibroblasts handled with the GPHCFD hydrogels exhibited important downregulation of genes associated to ERS capabilities, together with unfolded protein binding (GO:0051082), endoplasmic reticulum unfolded protein response (GO:0030968), and protein folding chaperone (GO:0044183) (Fig. 5B). These findings confirmed that the GPHCFD hydrogel may inhibit extreme ERS within the native fibroblasts of diabetic wounds, thereby selling fibroblast proliferation and migration.
We additional investigated the impact of the GPHCFD hydrogel on fibroblast collagen secretion. Masson’s trichrome was used to evaluate collagen formation on the diabetic wound surfaces of the 4 teams (no hydrogel remedy, GP, GPHCF, and GPHCFD hydrogel remedy) on days 8 and 15, and collagen thickness was statistically analyzed. Compared to the collagen layers within the different three teams, the GPHCFD hydrogel remedy group’s layer was the thickest on day 8 (Fig. 5C, D). By day 15, as a result of collagen within the GPHCFD hydrogel-treated group had begun to mature, the collagen layer within the GPHCF hydrogel-treated group was the thickest (Fig. 5C, S6). This discovering urged that the GPHCFD hydrogel considerably promoted collagen formation and maturation in fibroblasts. DCN is a attribute marker of fibroblasts. We used immunofluorescence to evaluate the expression of MCP-1 and the attribute ERS protein CHOP in diabetic wounds on day 3 within the untreated, GP, GPHCF, and GPHCFD hydrogel teams. In distinction to the remaining three teams, the GPHCFD group exhibited the bottom native MCP-1 expression degree and the bottom proportion of DCN+CHOP+ cells in diabetic wounds (Fig. 5E, G). Western blot evaluation was carried out to detect MCP-1 expression ranges on the wound websites throughout all teams. We equally discovered that the GPHCFD hydrogel group exhibited the bottom MCP-1 expression ranges in comparison with the opposite three teams (Fig. S7). This discovering indicated that the GPHCFD hydrogel may scale back the expression degree of MCP-1 and inhibit extreme ERS in fibroblasts on the wound website. The above outcomes indicated that GPHCFD hydrogel promoted tissue regeneration in wounds from diabetes by stopping the expression of MCP-1 in fibroblasts, suppressing ERS in fibroblasts, and selling collagen formation.
The GPHCFD hydrogel inhibits extreme ERS in diabetic wound fibroblasts by suppressing MCP-1 expression and promotes collagen formation. (A) GO enrichment evaluation bubble chart evaluation of wound healing-related capabilities in diabetic wound fibroblasts handled with the GPHCFD hydrogel. n = 3 per group. (B) GO enrichment circle plot evaluation of ERS-related capabilities in native fibroblasts from diabetic wound tissue handled with the GPHCFD hydrogel. n = 3 per group. (C) Masson’s trichrome to detect collagen formation in 4 teams of diabetic wounds: no hydrogel remedy and GP, GPHCF, and GPHCFD hydrogel remedy on days 8 and 15. 500 μm and 200 μm Scale bar. (D) Statistical analysis of collagen thickness on day 8. n = 6 per staff. (E) Immunofluorescence detection of MCP-1 expression ranges in diabetic wounds handled with the GPHCFD hydrogel (Crimson: DCN; Blue: nuclei; Inexperienced: MCP-1). Scale bar, 20 μm. (F) Immunofluorescence detection of DCN+CHOP+ cells within the native space of diabetic wounds handled with no hydrogel and GP, GPHCF, and GPHCFD hydrogels. Scale bar, 20 μm. (G) Statistical analysis of MCP-1’s imply fluorescence depth. n = 6 in every group. (H) The share of DCN+CHOP+ cells amongst DCN+ cells is statistically analyzed within the wound space. n = 6 per staff. NS: non-significant, *P < 0.05. The information is displayed as imply ± SD.
The GPHCFD hydrogel inhibited extreme ERS in NK cells, thereby suppressing irregular proliferation and inflammatory issue secretion in NK cells and selling NK cell maturation
The GO enrichment evaluation bubble chart reveals that genes considerably downregulated in native NK cells handled with the GPHCFD hydrogel in diabetic wound tissue are enriched within the following useful pathways: detrimental regulation of pure killer cell differentiation, ERS-related capabilities (PERK complicated, response to unfolded proteins, and endoplasmic reticulum membrane) (Fig. 6A). Volcano plot evaluation revealed that the expression of NK cell CCR2, ERS-related genes XBP1, ATF6B, IRE1, PERK, ATF6, ATF4, and inflammatory elements TNF-α and IFN-γ was considerably downregulated (Fig. 6B). To additional validate the GPHCFD hydrogel’s influence on NK cells in diabetic wounds, we used stream cytometry to detect NK cells in diabetic wounds on day 3 within the untreated, GP, GPHCF, and GPHCFD teams—particularly CD45+CD3−NK1.1+ cells. The GPHCFD group had the bottom proportion of CD3−NK1.1+cells among the many CD45 + cells when in comparison with the opposite three teams. This means that whereas the NK cells within the different three teams displayed important irregular proliferation, the GPHCFD group confirmed solely delicate irregular NK cell proliferation (Fig. 6C, E). The proportion of CD11b+CD27+ NK cells amongst CD3−NK1.1+cells in diabetic wound websites elevated most importantly among the many 4 teams within the GPHCFD group (Fig. 6C, F), however the proportion of CD11b+CD27−NK cells amongst CD3−NK1.1+ cells didn’t considerably alter (Fig. 6C, G). The GPHCFD hydrogel remedy considerably enhanced NK cell maturation towards the CD11b+CD27+ NK cells. The NK cells from diabetic wound websites within the untreated, GP, GPHCF, and GPHCFD teams have been then subjected to Western blotting evaluation. The expression ranges of CCR2 and ERS-related proteins p-IRE1 and XBP1 have been lowest within the wound-site NK cells of the GPHCFD hydrogel remedy group (Fig. 6H, I, S8). These outcomes demonstrated that the GPHCFD hydrogel may inhibit NK cell CCR2 expression, scale back extreme ERS ranges, suppress irregular NK cell proliferation and inflammatory issue secretion, and promote NK cell maturation. Mature NK cells can regulate wound irritation homeostasis, improve re-epithelialization pace, and enhance collagen deposition [38].
The GPHCFD hydrogel inhibits irregular proliferation and inflammatory issue secretion of NK cells by suppressing extreme ERS, thereby selling NK cell maturation. (A) GO enrichment evaluation bubble chart evaluation of ERS-related capabilities and NK cell differentiation capabilities in native NK cells of diabetic wound tissue handled with the GPHCFD hydrogel. n = 3 per group. (B) Volcano plot evaluation of ERS-related gene expression and inflammatory issue expression in native NK cells of diabetic wound tissue handled with the GPHCFD hydrogel. n = 3 per staff. (C) Stream cytometry was used to detect CD45+CD3−NK1.1+ NK cells from diabetic wounds within the untreated, GP, GPHCF, and GPHCFD teams. (D) Stream cytometry was used to detect CD11b+CD27+ NK cells and CD11b+CD27− NK cells in diabetic wounds from the untreated group, GP group, GPHCF group, and GPHCFD group, respectively. (E) The share of CD45+CD3−NK1.1+ cells inside CD45+ cells is statistically analyzed. n = 5 in every group. (F) Statistical evaluation of the share of CD11b+CD27+ NK cells amongst CD3−NK1.1+ cells. n = 5 per group. (G) The share of CD11b+CD27− NK cells amongst CD3−NK1.1+ cells. n = 5 per group. (H) Stream cytometry-sorted NK cells from diabetic wounds within the untreated, GP, GPHCF, and GPHCFD hydrogel remedy teams have been subjected to Western blotting. (I) A heatmap was used to statistically assess the degrees of CCR2, p-IRE1, and XBP1 protein expression in keeping with Western blotting information. n = 5 per staff. *P < 0.05, NS: non-significant. The information is displayed as imply ± SD.
The GPHCFD hydrogel promoted M1–M2 macrophage polarization by inhibiting ERS in macrophages
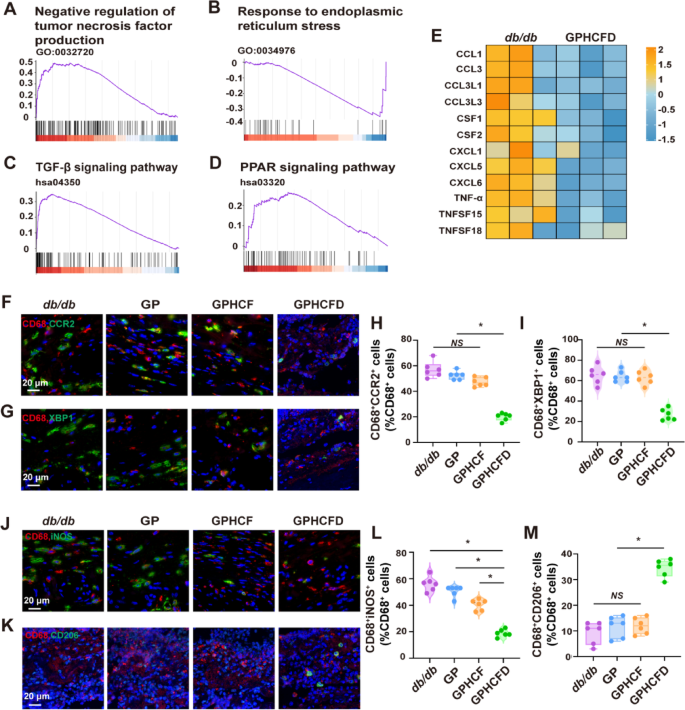
To additional discover the results of the GPHCFD hydrogel on native macrophages in diabetic wounds, we analyzed macrophage information from the untreated and GPHCFD teams utilizing single-cell sequencing. GSEA evaluation confirmed that within the diabetic wound tissue of the GPHCFD group, the negatively regulated genes associated to tumor necrosis issue manufacturing (inhibiting macrophage polarization to M1) have been considerably upregulated in native macrophages (Fig. 7A). The response-related genes of ERS have been considerably downregulated (Fig. 7B), whereas the KEGG pathway that promotes macrophage polarization in direction of the M2 sort, specifically the TGF-β signaling pathway-related genes, was considerably upregulated (Fig. 7C). The KEGG PPAR sign pathway-related genes that work together with ERS and macrophage polarization to M2 sort have been considerably up-regulated, additional indicating that the GPHCFD hydrogel can promote macrophage polarization to M2 sort by regulating ERS (Fig. 7D). The heatmap evaluation revealed that in contrast with these in untreated diabetic wound tissue, the degrees of inflammatory elements CCL1, CCL3, CCL3L1, CCL3L3, CSF1, CSF2, CXCL1, CXCL15, CXCL6, TNF-α, TNFSF15, and TNFSF18 have been considerably decreased in macrophages within the GPHCFD group (Fig. 7E). CD68 is a attribute marker of macrophages; thus, we used immunofluorescence to evaluate the extent of CCR2, the ERS biomarker XBP1, the M1 signature iNOS, and the M2 biomarker CD206 in CD68+ cells in diabetic wound tissues from the untreated, GP, GPHCF, and GPHCFD teams. The proportions of CD68+CCR2+ and CD68+XBP1+ cells have been considerably diminished (Fig. 7F-I), indicating that the GPHCFD hydrogel can inhibit macrophage CCR2 expression and scale back macrophage ERS. The proportion of CD68+iNOS+ cells considerably decreased, whereas that of CD68+CD206+ cells considerably elevated, indicating that the GPHCFD hydrogel promotes the M1–M2 conversion of diabetic wound macrophages (Fig. 7J-M). The above outcomes demonstrated that the GPHCFD hydrogel may inhibit the expression of CCR2 in native macrophages at diabetic wound websites, scale back extreme ERS in macrophages, and promote macrophage M1–M2 conversion.
The GPHCFD hydrogel promotes macrophage polarization towards the M2 sort by inhibiting ERS in macrophages. (A) GSEA plot evaluation of gene enrichment associated to the detrimental regulation of tumor necrosis issue manufacturing perform in native macrophages in diabetic wound tissue within the GPHCFD group. (B) The TGF-β signaling pathway in macrophages within the GPHCFD group was analyzed utilizing a GSEA plot. (C) GSEA plot evaluation of gene enrichment associated to the response to ERS perform in macrophages within the GPHCFD group. n = 3 per group. (D) GSEA plot evaluation of gene enrichment associated to the KEGG PPAR signaling pathway in macrophages within the GPHCFD group. n = 3 per group. (E) Heatmap evaluation of inflammatory issue expression in native macrophages from the untreated diabetic wound group and the GPHCFD hydrogel-treated group. n = 3 per group. (F) Immunofluorescence detection of CD68+CCR2+ cells in diabetic wounds within the untreated, GP, GPHCF, and GPHCFD hydrogel-treated teams. Scale bar, 20 μm. (G) Identification of CD68+XBP1+ cells within the diabetic wounds within the 4 teams by immunofluorescence. 20 μm Scale bar. (H) The share of CD68+CCR2+ cells amongst CD68+ cells is statistically analyzed. n = 6 every group. *P < 0.05, NS: non-significant. (I) Statistical evaluation of the proportion of CD68+XBP1+ cells amongst CD68+ cells. n = 6 in every group. (J) Immunofluorescence detection of CD68+ iNOS+ cells within the diabetic wounds within the 4 teams. Scale bar, 20 μm. (Okay) Immunofluorescence detection of CD68+CD206+ cells within the diabetic wounds within the 4 teams. 20 μm Scale bar. (L) The share of CD68+iNOS+ cells amongst CD68+ cells is statistically analyzed. (M) Statistical evaluation of the proportion of CD68+CD206+ cells amongst CD68+ cells. n = 6 per staff. *P < 0.05, NS: non-significant. The information is displayed as imply ± SD.